Dry powder inhalant matched with pollen-shaped saccharide carrier as well as preparation and application methods thereof
A dry powder inhaler, sugar technology, applied in pharmaceutical formulations, powder delivery, drug delivery and other directions, can solve the problems of inability to use pulmonary drug delivery, low drug carrying capacity, insufficient practicability, etc., to improve the respiratory tract transmission effect, Better efficacy and better practicality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
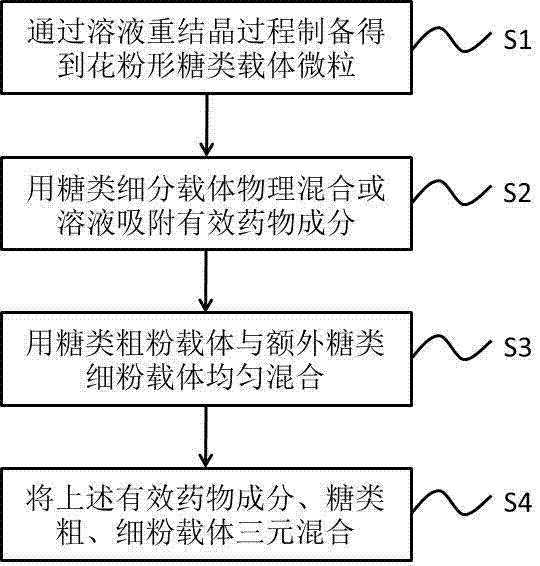
[0037] This embodiment provides a dry powder inhaler formulated with a pollen-shaped sugar carrier, which contains a ternary mixed component, including 0.01-2 parts of active pharmaceutical ingredients (the median aerodynamic diameter is preferably 0.5-5 µm), 0- 20 parts of coarse sugar powder carrier (median aerodynamic diameter is preferably 10-100µm) and 0-20 parts of fine sugar powder carrier (median aerodynamic diameter is preferably 1-10µm), wherein the coarse sugar The powder carrier and / or sugar fine powder carrier adopts prepared pollen-shaped sugar carrier particles (the particle surface is rough, the interior is porous, and the BET pore surface area is 10-60m 2 / g), and the density of the sugar coarse powder carrier is preferably 1-3 times of the sugar fine powder carrier density, and the particle diameter of the sugar coarse powder carrier is preferably 5-10 times of the sugar fine powder carrier particle diameter; The fine powder distribution ratio (FPF) of the dr...
Embodiment 2
[0046] This embodiment provides a method for preparing a dry powder inhaler combined with a pollen-shaped carbohydrate carrier, comprising the following steps:
[0047] S1: Mix 50 grams of amorphous lactose particles prepared by spray drying with 500 mL of ethanol, stir for 30 minutes, filter and remove the supernatant, and sort the air-dried precipitated particles into flower-shaped sugars with a median particle size of 7 microns by air separator A fine powder carrier and a flower-shaped sugar coarse powder carrier with a median particle size of 20 microns;
[0048] S2: Finely grind the commercially available budesonide micropowder to obtain an active pharmaceutical ingredient with a median diameter of 1.5 microns, and physically mix 2 parts of the sugar fine powder carrier with 0.05 part of the active pharmaceutical ingredient in proportion for 5 minutes ;
[0049] S3: additionally, physically mix 5 parts of the saccharide fine powder carrier with 13 parts of the saccharide...
Embodiment 3
[0053] This embodiment provides a method for preparing a dry powder inhaler combined with a pollen-shaped carbohydrate carrier, comprising the following steps:
[0054] S1: Mix 10 grams of amorphous lactose particles prepared by supersaturated solution precipitation with 100 mL of ethanol, stir for 15 minutes, filter and remove the supernatant, and sort the air-dried precipitated particles through an airflow separator to obtain flower-shaped particles with a median particle size of 8 microns Sugar fine powder carrier and flower-shaped sugar coarse powder carrier with a median particle size of 30 microns;
[0055] S2: finely grinding the commercially available tiotropium bromide micropowder to obtain an active pharmaceutical ingredient with a median diameter of 2 microns;
[0056] S3: physically mixing 5 parts of the fine sugar powder carrier with 15 parts of the fine sugar powder carrier in proportion for 60 minutes;
[0057] S4: Physically mix the above-mentioned active drug...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com