Pre-metered dry powder inhaler for moisture-sensitive medicaments
A dry powder inhaler, a predetermined amount of technology, used in inhalers, medical containers, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
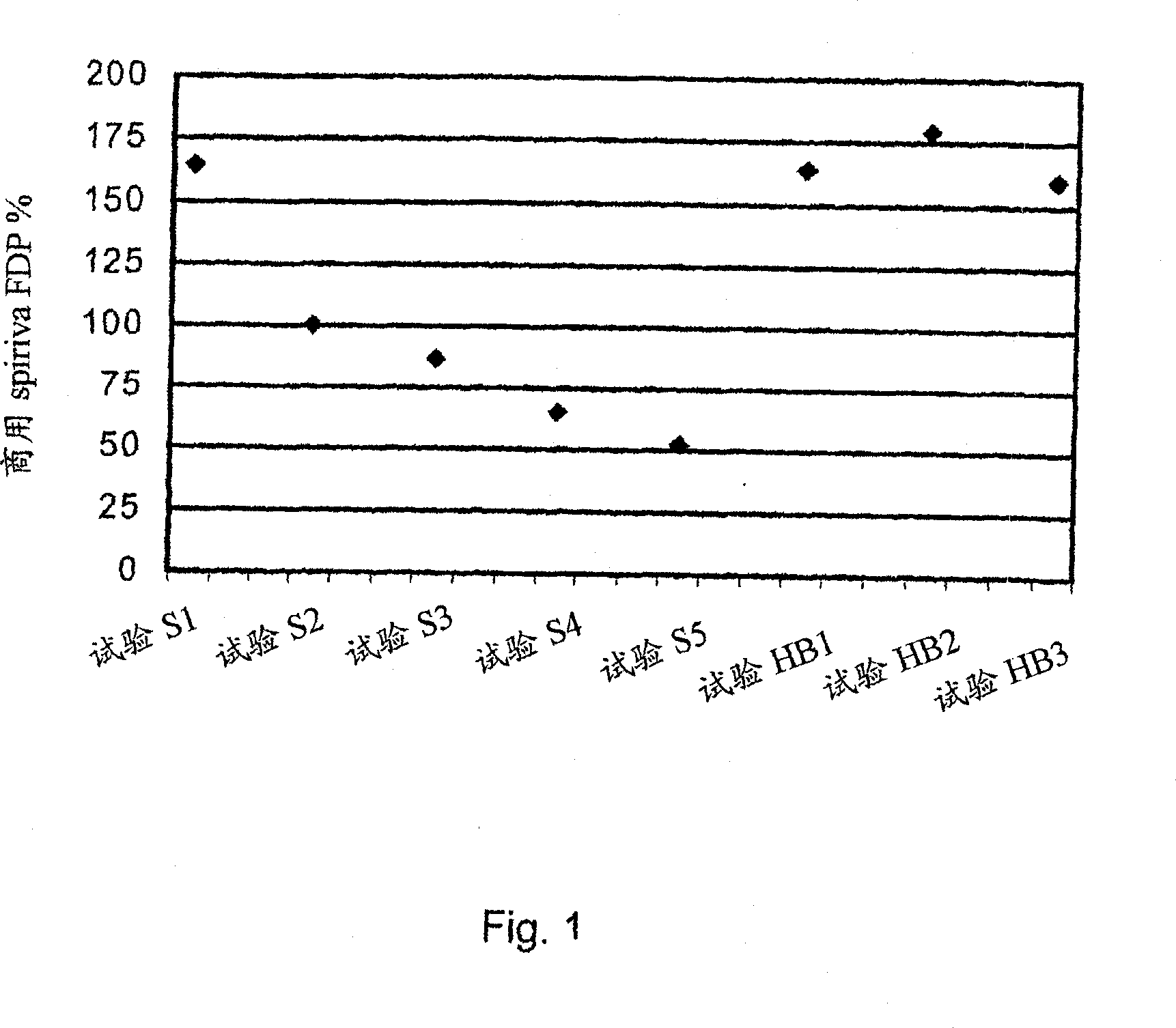
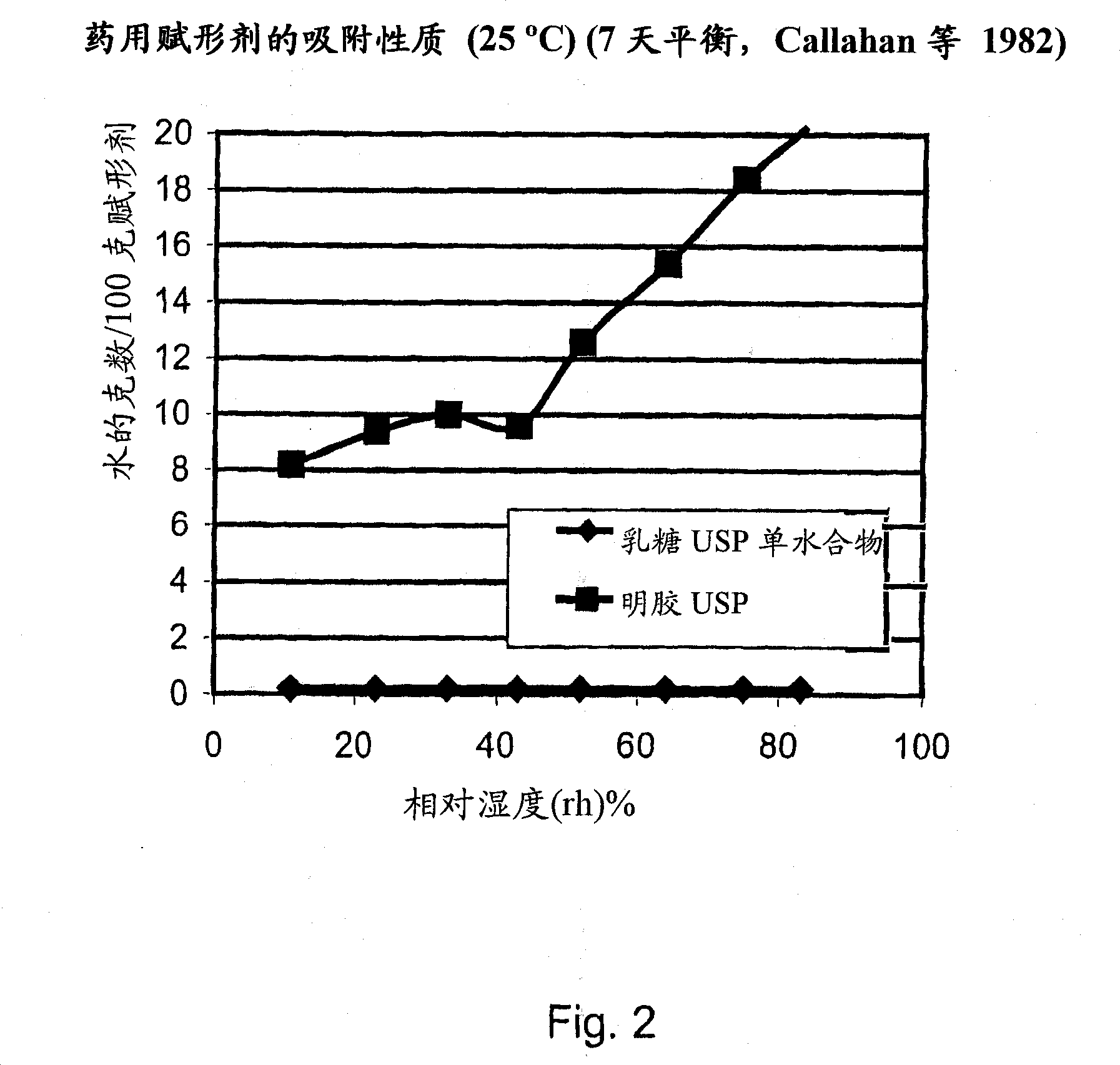
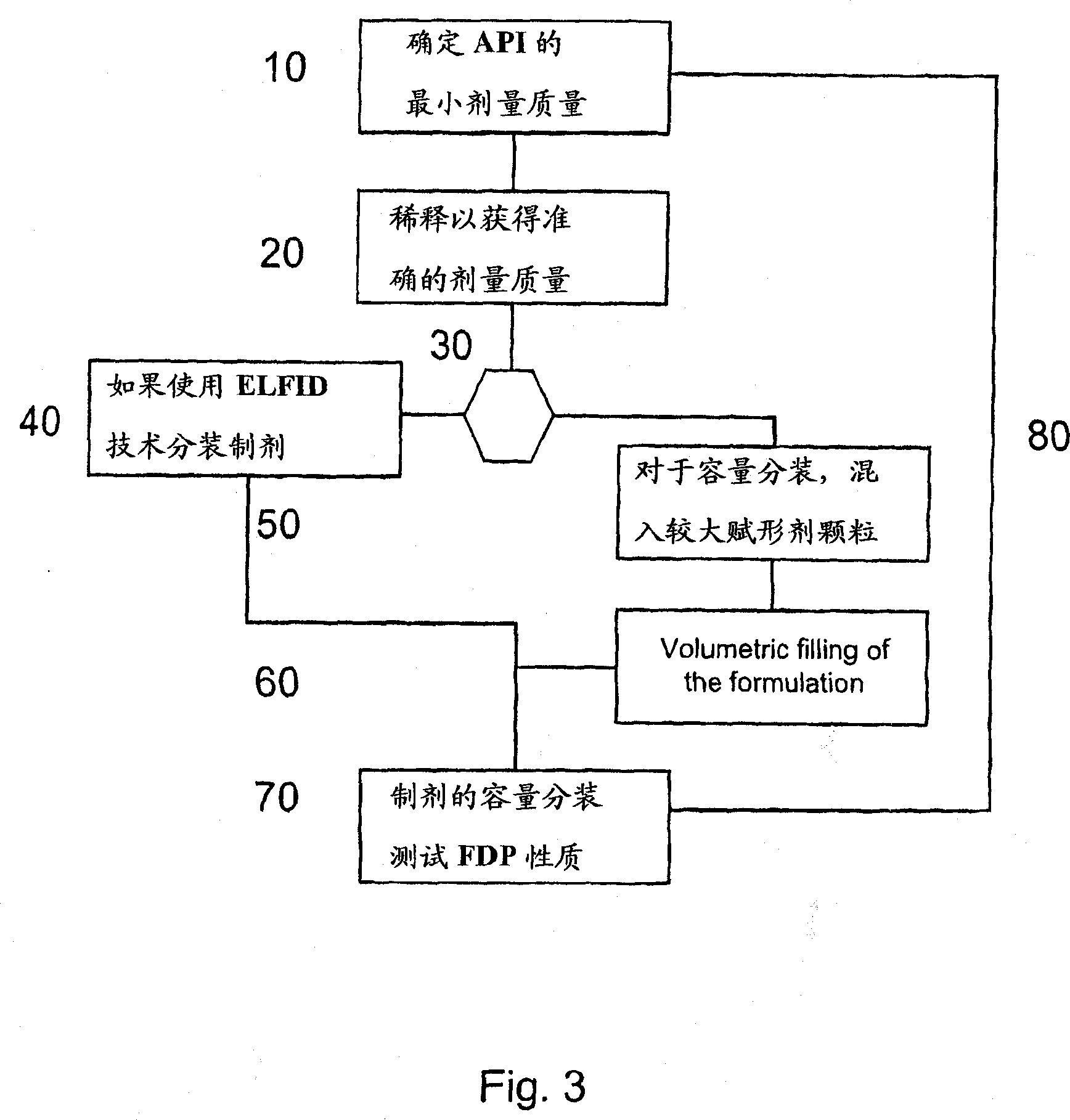
[0029] The present invention relates to DPI loaded with a moisture sensitive drug, preferably comprising tiotropium, and describes dose release to achieve high levels of released FPD. Preferably, the DPI is a predetermined amount. Additionally, the present invention addresses how such sensitive drugs can be protected from the moment of dose formation and sealing to the moment the user inhales the selected dose, throughout all stages of storage, transport, distribution, restorage and final administration. No moisture problem. Suitable dry powder inhalers for moisture sensitive medicaments are also disclosed.
[0030] The present invention discloses a dry, moisture-proof, ready-to-load and seal container containing a dose of tiotropium in a high FPD formulation containing at least one excipient. The term "tiotropium" is a generic term for all active forms thereof, including pharmaceutically acceptable salts (especially...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com