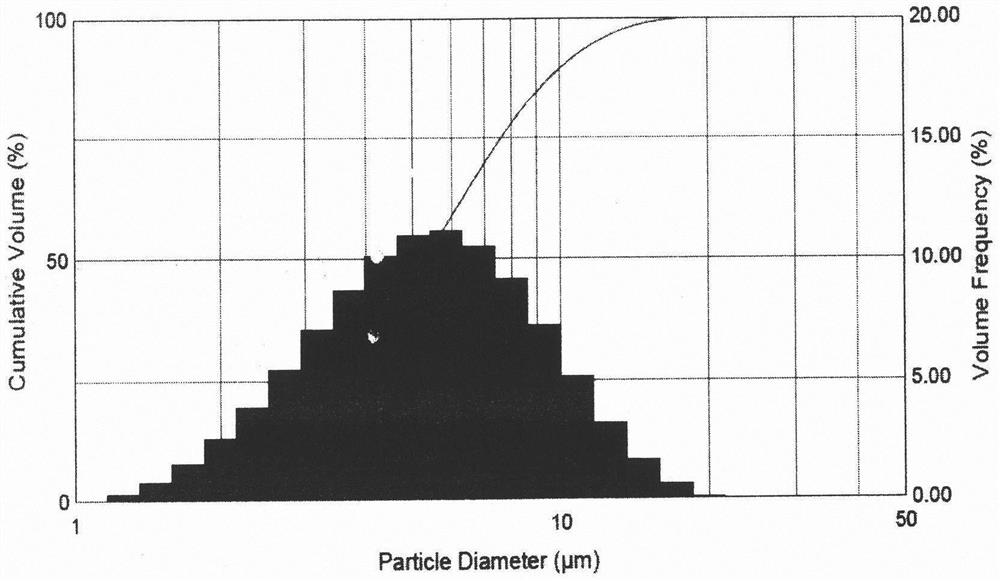
Aerosol inhalant and preparation method thereof
A technology of nebulized inhalant and atomizing device, which is applied in the field of medicine to achieve the effect of simple production process, small particle size and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
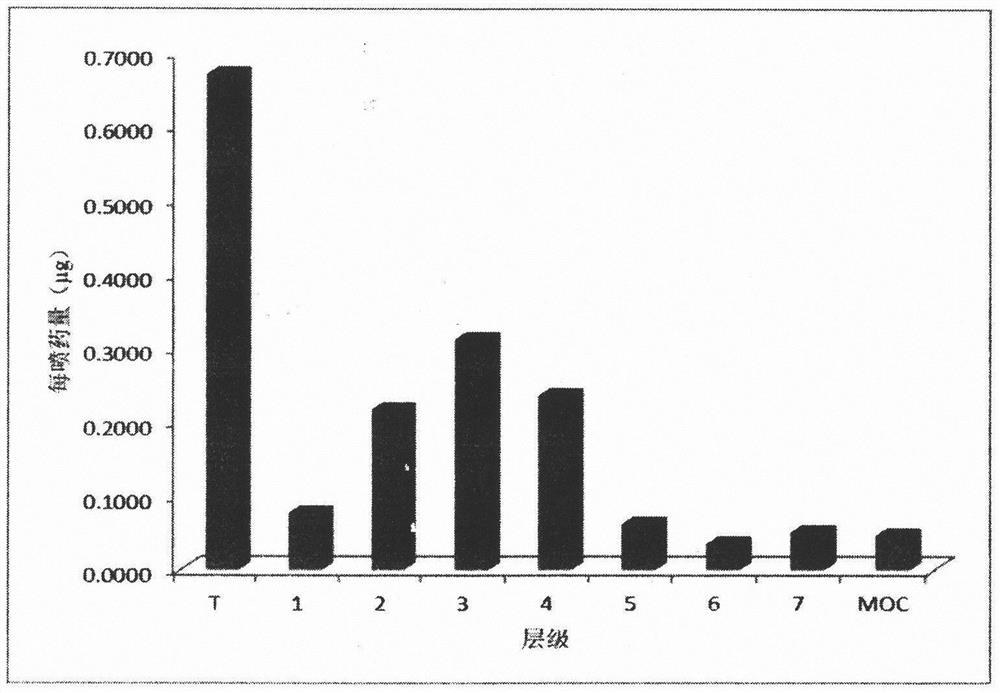
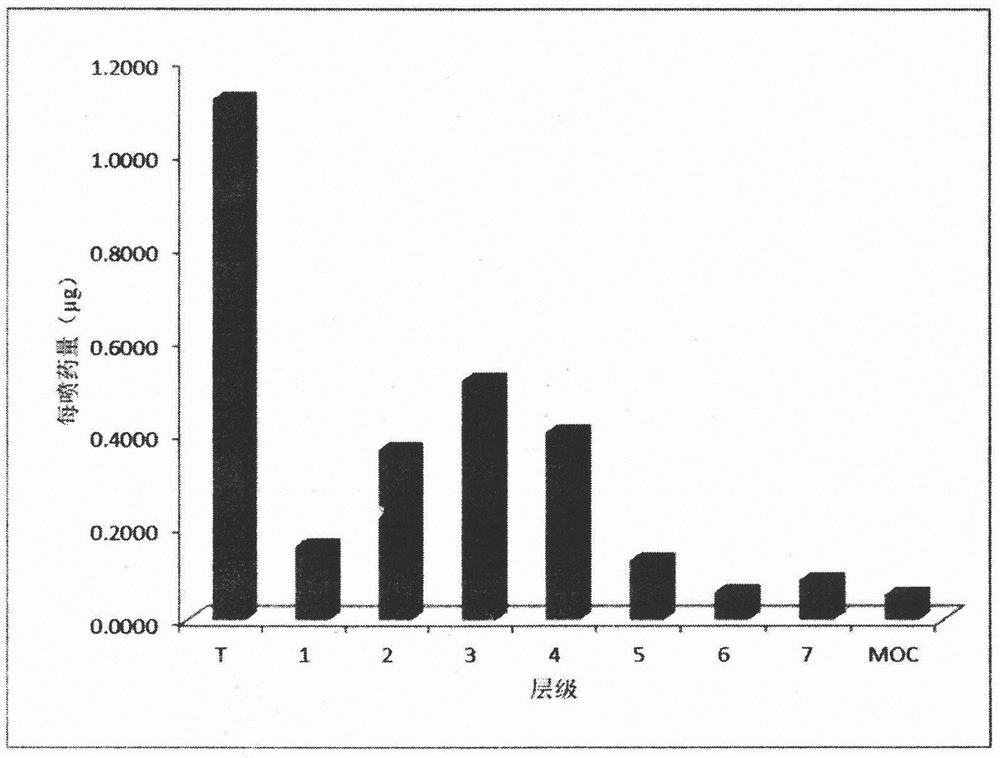
Image
Examples
Embodiment 1
[0052] Preparation of sample 1 and sample 2 inhalation solution: Weigh 50% benzalkonium chloride and Tween-80 of the prescription in Table 1 with a weighing bottle, dissolve in 95% ethanol of the prescription in Table 1 in three times and transfer to the prepared solution. In the weighed 100ml volumetric flask, add umeclidinium bromide and vilanterol triphenylacetate in the recipe quantities in Table 1, and dissolve by ultrasonic. Then add the prescription amount of edetate disodium dihydrate dissolved in an appropriate amount of pure water, and then ultrasonically dissolve, and make up the volume to 100ml with pure water. Both samples 1 and 2 are clear solutions, and the measured content results are shown in Table 2, indicating that both umeclidinium bromide and vilanterol triphenylacetate have been completely dissolved in the dilute ethanol solution containing Tween-80 .
[0053] Table 1 Recipe for sample 1 and sample 2 inhalation solutions
[0054] Element Sam...
Embodiment 2
[0058] Weigh the 50% benzalkonium chloride and Tween-80 of the recipe amount in Table 3 with a weighing bottle, dissolve and transfer to the weighed 100ml volumetric flask with the 95% ethanol of the recipe amount in Table 3 in three times, and then add The umeclidinium bromide and vilanterol triphenylacetate of recipe quantity in table 3, ultrasonic dissolving. Add the disodium edetate dihydrate of the recipe quantity that dissolves with an appropriate amount of pure water again, and ultrasonically dissolve again, and make up to 100ml with pure water. Sample 3, sample 4 and sample 5 are all clear solutions, and the measured content results are shown in Table 4, indicating that umeclidinium bromide and vilanterol triphenylacetate have been completely dissolved in dilute solution containing Tween-80. in ethanol solution.
[0059] Table 3 Recipe for sample 3, sample 4 and sample 5 inhalation solutions
[0060]
[0061] Table 4 Assay results of sample 3, sample 4 and sample ...
Embodiment 3
[0064] Weigh the 50% benzalkonium chloride and Tween-80 of the recipe in Table 5 with a weighing bottle, dissolve and transfer to the weighed 100ml volumetric flask with the 95% ethanol of the recipe in Table 5 in three times, and then add in Table 5. The prescribed amounts of umeclidinium bromide and vilanterol triphenylacetate were dissolved by ultrasonic. Then add the prescribed amount of edetate disodium dihydrate dissolved in an appropriate amount of pure water, then dissolve by ultrasonic, and make up to 100ml with pure water. Sample 3, sample 4 and sample 5 are all clear solutions, and the measured content results are shown in Table 6.
[0065] Table 5 Recipe for sample 6, sample 7 and sample 8 inhalation solutions
[0066] Element Sample 6 Sample 7 Sample 8 umeclidinium bromide 21mg 21mg 21mg vilanterol triphenylacetate 11mg 11mg 11mg Disodium Edetate Dihydrate 11mg 11mg 11mg 50% benzalkonium chloride 20mg 20mg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com