Simvastatin dry powder inhalant and preparation method thereof
A technology of dry powder inhaler and simvastatin, which is applied in the field of simvastatin dry powder inhaler and its preparation, can solve the problems of mucous membrane irritation and inability to completely eliminate the greenhouse effect, and achieve increased deposition, improved powder fluidity and Good drug release ability and atomization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
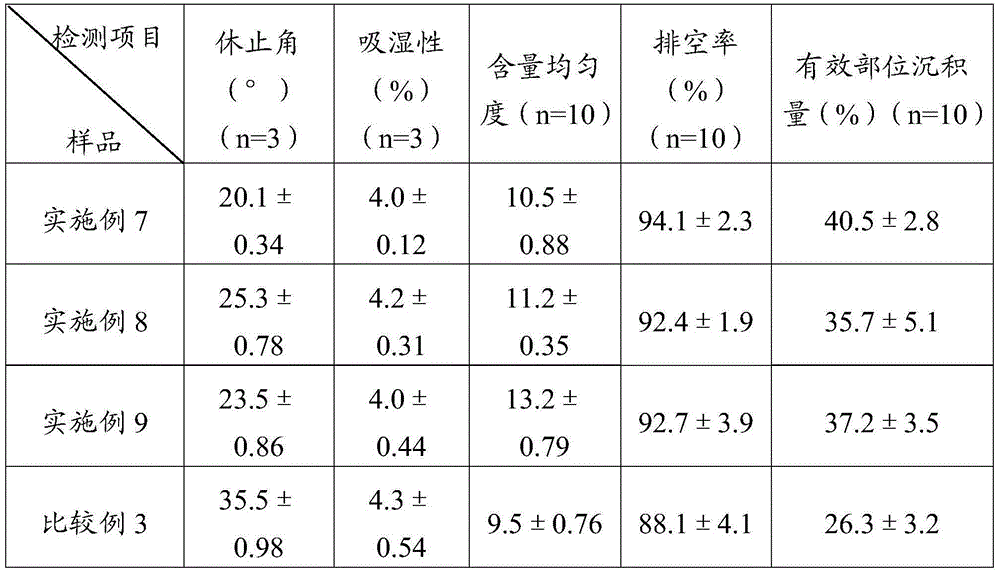
Embodiment 1
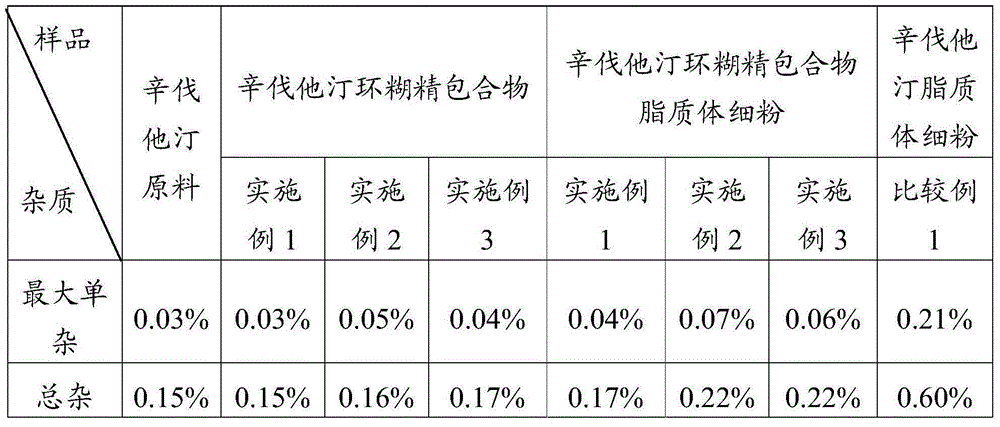
[0045] Weigh 100 g of simvastatin and 322 g of sulfobutyl ether-β-cyclodextrin, mix them uniformly in a grinder, add 25% ethanol aqueous solution, grind for 1 hour, and dry at 45° C. to obtain simvastatin cyclodextrin inclusion compound.
[0046] Adopt the SD-1000 spray drier of Japanese EYELA company to prepare simvastatin liposome complex fine powder, the above-mentioned prepared simvastatin cyclodextrin inclusion complex 50g, soybean lecithin 60g are miscible in 2L isopropanol, Spray drying is carried out after filtration, the adopted process parameters are: inlet temperature 100°C, spray speed 2.0mL min -1 , pump pressure 160KPa, air flow 0.7m 3 min -1 , the outlet temperature was 80° C., and the fine powder of simvastatin liposome complex was prepared.
Embodiment 2
[0048] Weigh 100 g of simvastatin and 358 g of hydroxypropylmethyl-β-cyclodextrin, mix them in a grinder, add 25% ethanol aqueous solution, grind for 1 hour, and dry at 45°C to obtain simvastatin cyclodextrin inclusion complex.
[0049] Adopt the SD-1000 spray drier of Japanese EYELA company to prepare simvastatin liposome complex fine powder, the above-mentioned prepared simvastatin cyclodextrin inclusion compound 50g, egg yolk phospholipid 70g are mixed and dissolved in 2L ethanol, after filtering For spray drying, the imported process parameters are as follows: temperature is 85°C, spray speed is 2.0mL min -1 , pump pressure 160KPa, air flow 0.7m 3 min -1 , the outlet temperature was 75° C., and the fine powder of simvastatin liposome complex was prepared.
Embodiment 3
[0051] Weigh 100 g of simvastatin and 271 g of β-cyclodextrin, mix them in a grinder, add 25% aqueous ethanol, grind for 1 hour, and dry at 45° C. to obtain simvastatin cyclodextrin inclusion compound.
[0052] Adopt the SD-1000 spray dryer of Japanese EYELA company to prepare simvastatin liposome complex fine powder, the simvastatin cyclodextrin inclusion compound 50g prepared above, phosphatidylcholine 70g are dissolved in 2L ethyl acetate solution In the process, spray drying is carried out after filtration, the adopted process parameters are: the inlet temperature is 90°C, and the spray speed is 2.0mL·min -1 , pump pressure 160KPa, air flow 0.7m 3 min -1 , the outlet temperature was 70° C., and the fine powder of simvastatin liposome complex was prepared.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com