Fudosteine inhalant composition
A technology for dry powder and inhalation of fodosteine, which is applied in the field of medicine, can solve the problem of no fodosteine inhalation drug being marketed and other problems, and achieves the effect of improving the fluidity, good fluidity and smooth surface of the powder.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] In this embodiment, the HLSH2-6A wet mixing granulator of AVIC Beijing Aeronautical Manufacturing Engineering Research Institute is used.
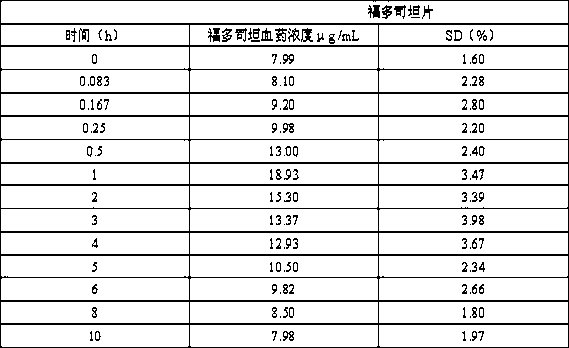
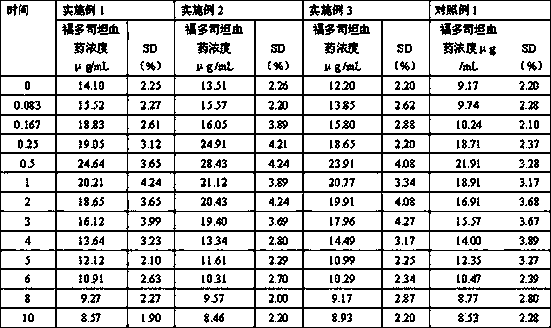
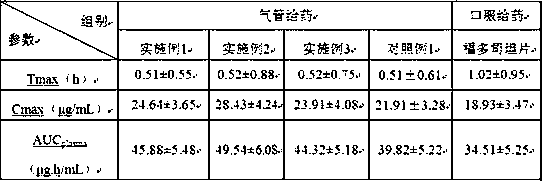
[0027] Put 10 g of fudosteine with a D90 of 2 μm and 30 g of lactose with a D90 of 50 μm in a high-shear mixer granulator, and mix evenly with a stirring blade at a speed of 300 rpm and a chopping knife at a speed of 900 rpm. Mix for 300 s to prepare fudosteine dry powder inhaler, fill the spray-dried powder into No. 5 capsules to make 400 servings, each containing 80 mg fudosteine dry powder inhaler.
Embodiment 2
[0029] In this embodiment, the HLSH2-6A wet mixing granulator of AVIC Beijing Aeronautical Manufacturing Engineering Research Institute is used.
[0030] Put 13.2 g of fudosteine with a D90 of 3.5 μm and 26.8 g of lactose with a D90 of 50 μm in a high-shear mixer granulator and mix evenly. After 300 s, the fudosteine dry powder inhaler was prepared, and the spray-dried powder was filled in No. 5 capsules to prepare 550 servings, each containing 60 mg of fudosteine dry powder inhaler.
Embodiment 3
[0032] In this embodiment, the HLSH2-6A wet mixing granulator of AVIC Beijing Aeronautical Manufacturing Engineering Research Institute is used.
[0033] Put 16 g of fudosteine with a D90 of 5 μm and 24 g of lactose with a D90 of 50 μm in a high-shear mixer granulator, and mix evenly with a stirring blade at 300 rpm and a chopping knife at 900 rpm. After 300 s, the fudosteine dry powder inhaler was prepared, and the spray-dried powder was filled in No. 5 capsules to prepare 600 servings, each containing 50 mg of fudosteine dry powder inhaler.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com