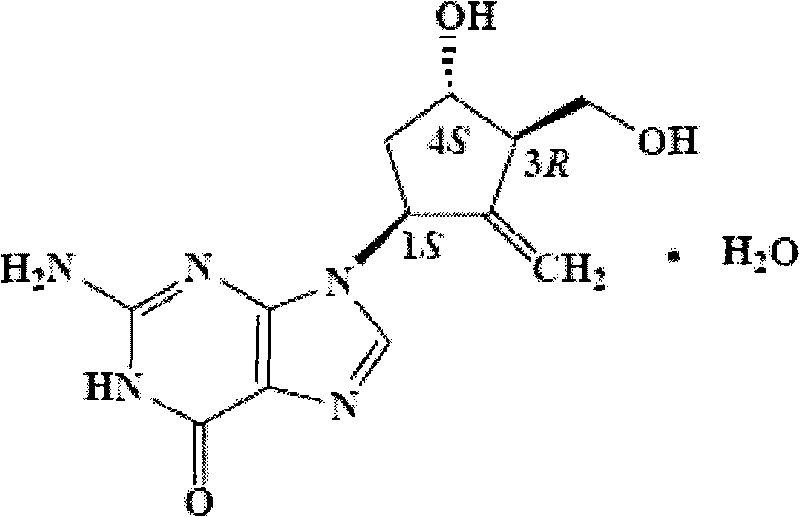
Entecavir soft capsule
A technology of entecavir and soft capsules, applied in the field of entecavir soft capsules, can solve problems such as low penetration rate, achieve low penetration rate, ensure rapid release, and stabilize the composition system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
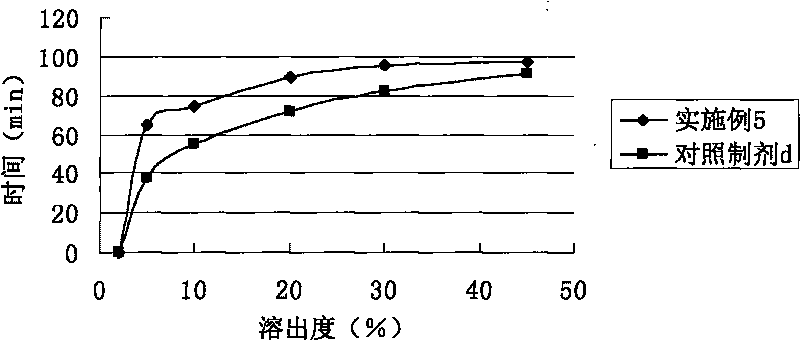
Image
Examples
Embodiment 1
[0027] Weigh 1975.0 mg of polyethylene glycol 400, heat it in a water bath to 70°C and keep it warm, then add 5.0 mg of entecavir into it, and stir until dissolved. Form a solution ①, then add 20mg of povidone K30 into the solution ①, stir and dissolve. If adding preservatives, add preservatives and stir to dissolve. Serve the content composition of entecavir soft capsule, inject this composition in the soft capsule preparation machine, regulate the injection rate of machine, make the content composition of entecavir soft capsule of every soft capsule 0.2g, entecavir active component content is 0.5mg.
Embodiment 2
[0029] Prepare the following soft capsules with the following composition by synchronously synchronizing according to embodiment 1, just as the povidone consumption of solubilizing agent is different or increases preservative:
[0030] 2-a Component Content (mg / capsule)
[0031] Entecavir 0.5
[0032] Polyethylene glycol 400 197.0
[0033] Povidone K30 2.5
[0034] Total 200.0
[0035] 2-b Component Content (mg / capsule)
[0036] Entecavir 0.5
[0037] Polyethylene glycol 400 198.5
[0038] Povidone K30 1.0
[0039] Total 200.0
[0040] 2-c Component Content (mg / capsule)
[0041] Entecavir 0.5
[0042] Polyethylene glycol 400 196.5
[0043] Povidone K30 3.0
[0044] Total 200.0
[0045] 2-d Component Content (mg / capsule)
[0046] Entecavir 0.5
[0047] Polyethylene glycol 400 196....
Embodiment 3
[0052]Weigh 1969 mg of polyethylene glycol 400, heat it in a water bath to 70°C and keep it warm, then add 5.0 mg of entecavir into it, and stir until dissolved. into a solution ①, then add 20 mg of povidone K30 into the solution ①, stir, dissolve, and form a solution ②, add poloxamer 188 6 mg, stir at a high speed, and stir until it becomes a milky white liquid. If preservatives are added, add preservatives, Stir, dissolve. Serve the content composition of entecavir soft capsule, this composition is injected in the soft capsule preparation machine, regulate the injection rate of machine, make the content composition of entecavir soft capsule of every soft capsule 0.2g, entecavir active ingredient content is 0.5mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com