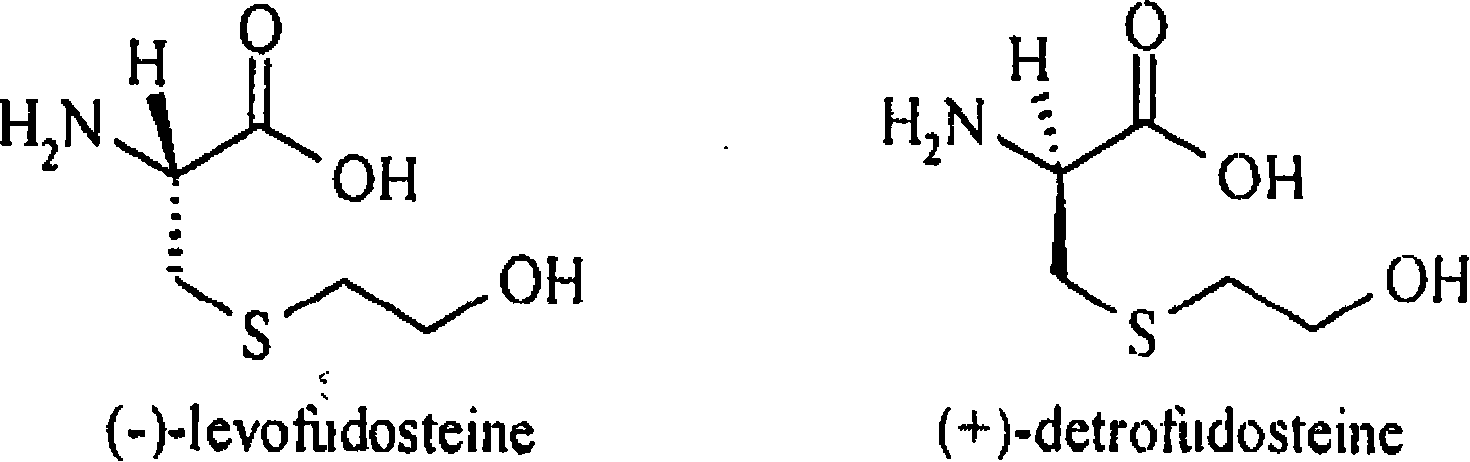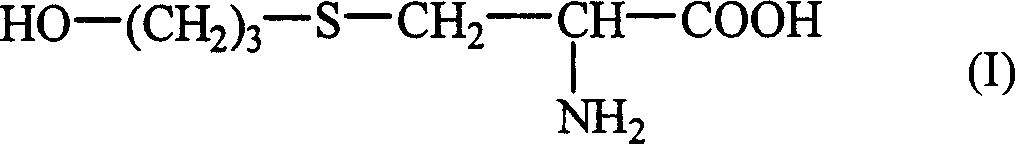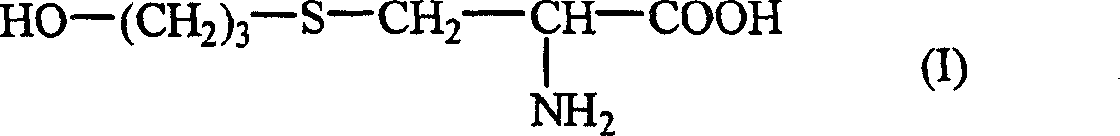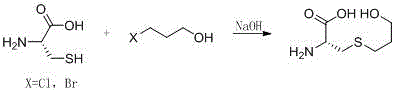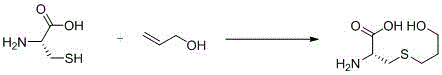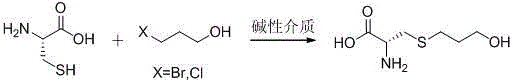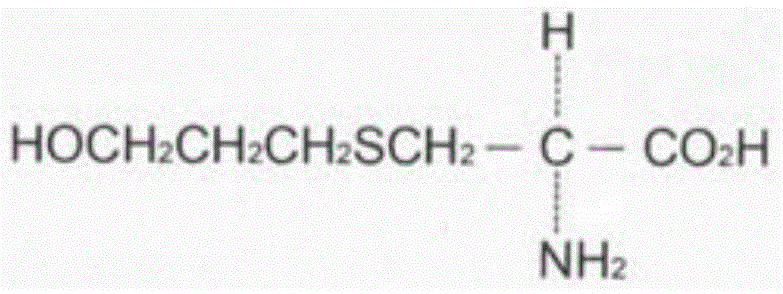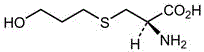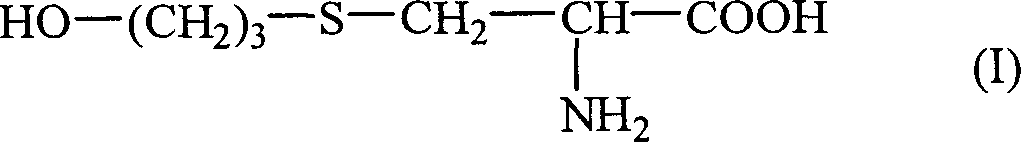Patents
Literature
51 results about "FUDOSTEINE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
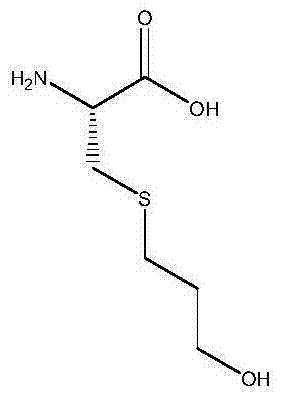
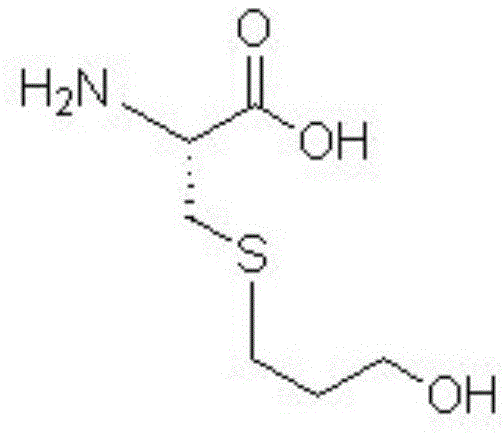
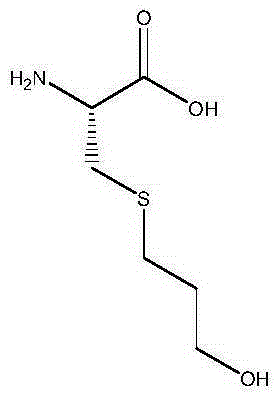
Fudosteine is a medicine available in a number of countries worldwide. A list of US medications equivalent to Fudosteine is available on the Drugs.com website.
Fudosteine synthesis method
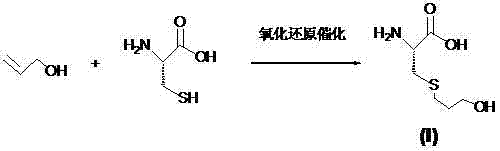
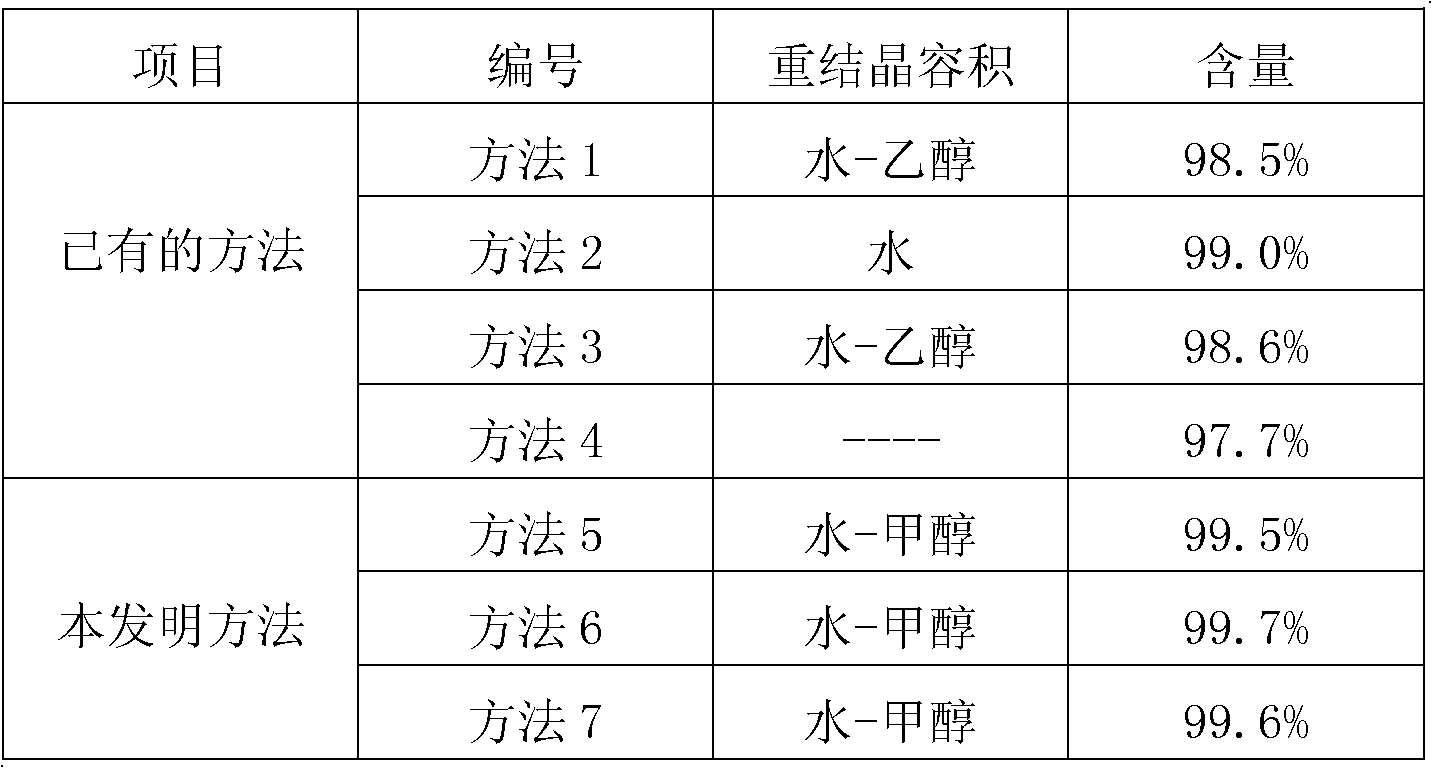
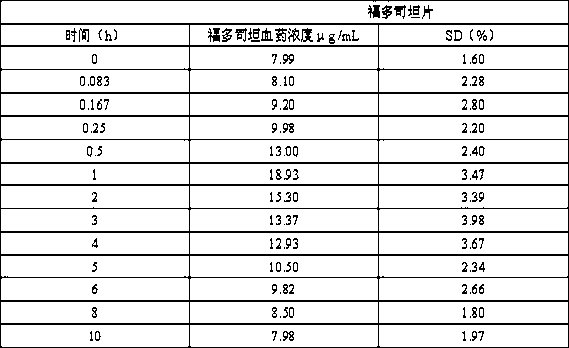
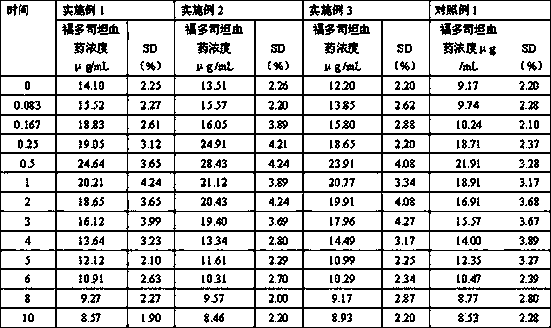
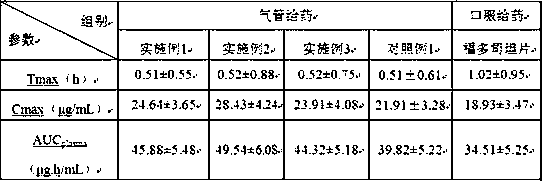
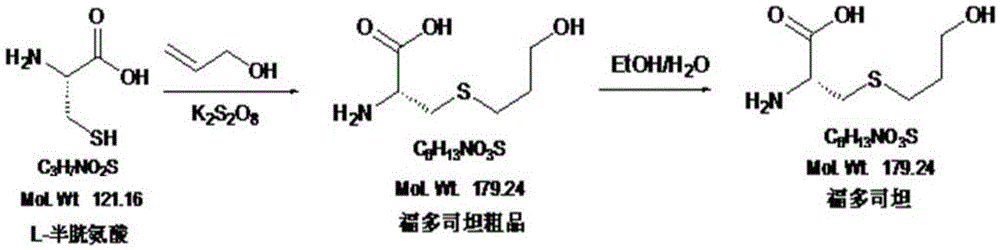
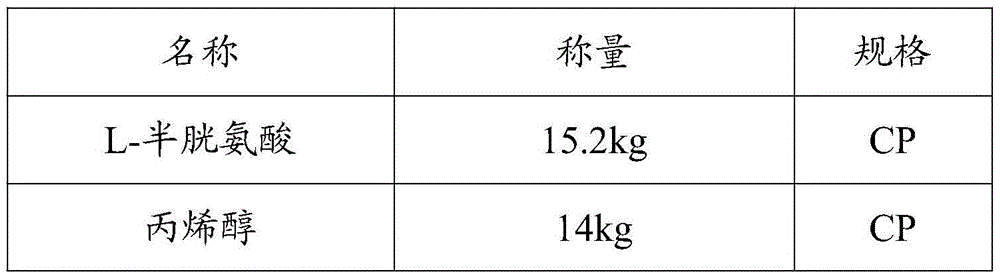
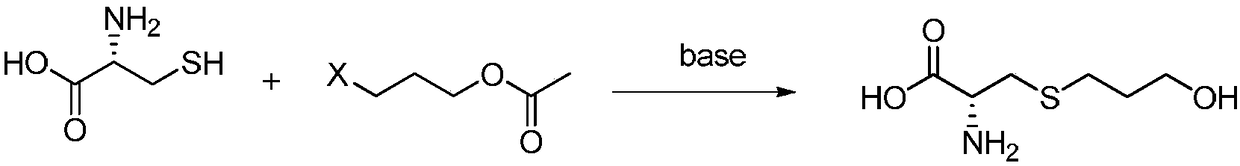
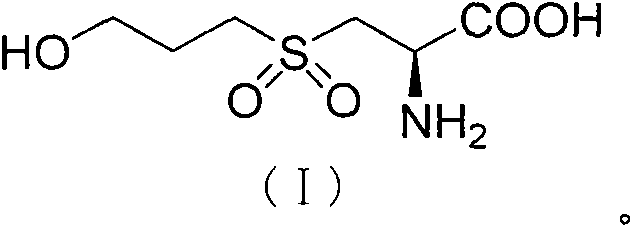
The invention mainly discloses a fudosteine synthesis method which comprises the following steps: by using a redox system to catalyze free radical reaction, mixing L-cysteine used as an initial raw material with a proper amount of purified water, and dissolving by stirring at a certain temperature; adding propenol, further adding transition metal or salt thereof, gradually adding water-soluble peroxide dropwisely at a temperature of 0-50 DEG C, keeping the temperature for some time, filtering to remove insoluble substances, and performing reduced pressure recovery on the filtrate to recover the unreacted solvent and a proper amount of water; after the recovery is finished, adding ethanol to perform crystallization; and performing secondary crystallization on the obtained solids with purified water, and drying to obtain the finished product fudosteine (I). The synthesis method disclosed by the invention is mild in condition, high in product yield (no less than 90%), high in content (no less than 99%) and low in inorganic salt residue (no more than 0.01%), thus being a very competitive synthetic route.
Owner:ZHEJIANG GUOBANG PHARMA
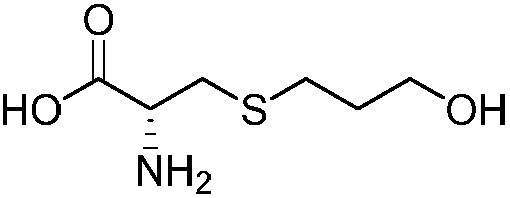
Method for decomposing chiral mobile phase additive RP-HPLC of fudosteine enantiomer
InactiveCN101161642AFast and accurate separabilityFast and Accurate DeterminationOrganic compound preparationOptically-active compound separationFUDOSTEINEHplc method
The present invention belongs to analytical chemistry field, and relates to separation and determination of Fudosteine and the enantiomer thereof (impurity). The present invention adopts RP-HPLC method, and adds a chiral metal synergist into a chromatogram flow phase system to form a tri-diastereoisomer coordination compound, because the obtained diastereoisomer coordination compound has stable structure with energy difference and capability to carry out three-dimensional selective absorption and repulsion reaction with the fixed phase, the two enantiomers can be separated from each other. The method can separate and determine Fudosteine and the enantiomer thereof (impurity), thereby making the quality of both Fudosteine and the agent containing Fudosteine controllable.
Owner:NANJING MEDICAL UNIV
Medicinal compsns.
Medicinal compositions characterized by containing fudosteine and an antipyretic / analgesic. These medicinal compositions are medicinal compositions to be used for common cold, etc. which have improved sputum-removal and antiinflammatory effects.
Owner:SS PHARMA CO LTD
Preparation method of high-purity fudosteine
ActiveCN104418779ASteady improvement in qualityGood effectSulfide preparation1-PropanolReaction step
The invention provides a preparation method of a novel antitussive phlegm-eliminating drug high-purity fudosteine, wherein the preparation method includes the steps: (1) synthesizing an intermediate 3-bromo-1-propanol; (2) carrying out a reaction of cysteine hydrochloride with 3-bromo-1-propanol to obtain a crude product; and (3) carrying out crystallization refining on the obtained crude product to obtain the high-purity fudosteine. The domestic cheap easily-available raw materials of cysteine hydrochloride, hydrobromic acid and 1,3-propylene glycol materials are utilized, monitoring of a gas chromatograph, a liquid chromatograph and a refractometer is used for tracking a catalytic reaction process and a reaction terminal point. The preparation method has the advantages of less reaction steps, high selectivity, low pollution, low cost and stable quality, and is suitable for industrialized production.
Owner:TOPFOND PHARMA CO LTD
Synthesis method of fudosteine
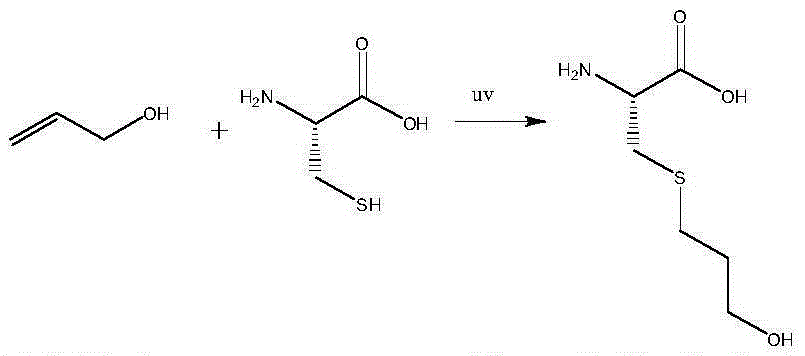
ActiveCN104326954AMild reaction conditionsHigh yieldSulfide preparationSynthesis methodsUltraviolet lights
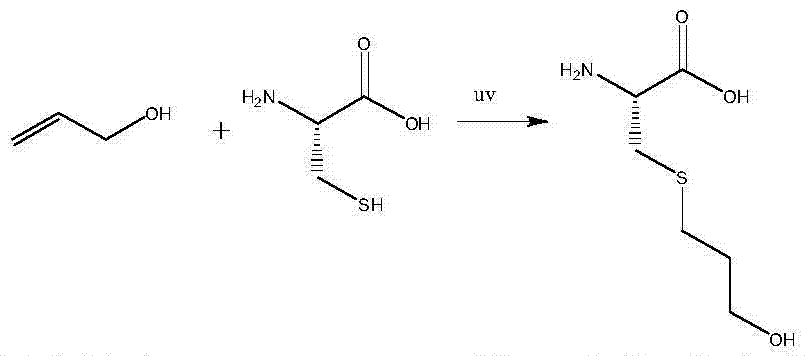
The invention discloses a synthesis method of fudosteine. The synthesis method comprises the following steps: in a glacial acetic acid-water system serving as a solvent, initiating a free radical reaction between L-cysteine and allyl alcohol which serve as starting raw materials at 40 DEG C by ultraviolet light; then, dropwise adding a certain amount of ethanol solution, cooling to 20 DEG C, and filtering to obtain a crude product of fudosteine; recrystallizing the crude product of fudosteine with 5-100% ethanol; filtering for the second time and drying to finally obtain high-purity fudosteine. The synthesis method of fudosteine is mild in synthesis condition and high in product yield which is higher than or equal to 92%; HPLC purity is higher than or equal to 99% and the maximum net contamination is less than or equal to 0.1%. The synthesis method of fudosteine can meet the demands of the market on fudosteine bulk drug.
Owner:YICHANG HEC CHANGJIANG PHARMA CO LTD
Drug preparations
Drug preparations characterized by containing fudosteine and an antitussive. These preparations are drugs to be used for common cold, etc. which have improved antitussive and sputum-removal effects.
Owner:SS PHARMA CO LTD
Method for preparing high-purity Fudosteine
ActiveCN102180820AHigh purityMild conditionsOrganic chemistryOrganic compound preparationFUDOSTEINEPurification methods
The invention discloses a method for preparing high-purity Fudosteine. The method comprises the following steps of: dissolving 100 weight parts of Fudosteine crude product with 40 to 600 weight volume parts of water at the temperature of between 100 and 10 DEG C; adding active carbon in an amount which is 0.005 to 0.05 times weight volume of the using amount of the water, and performing adsorption and decoloring; filtering; adding 4 to 6,000 weight volume parts of methanol, and crystallizing at the temperature of between -25 and 15 DEG C; and filtering and drying to obtain the pure Fudosteine product. The Fudosteine prepared by the method has the high performance liquid chromatography (HPLC) purity of over 99.5 percent and has the advantages of mild condition for purifying the Fudosteine, high yield, high purity, suitability for industrialized production and safe and controllable quality.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Fudosteine injection and preparation method thereof
The invention relates to medicinal preparations, specifically to a fudosteine injection. The fudosteine injection is a stable pharmaceutical composition composed of the active component fudosteine and pharmaceutically acceptable adjuvant materials which comprises excipient, a pH value regulating agent, an osmotic pressure regulating agent, injection water and the like. According to the invention, since above-mentioned carriers are used and scientific preparation is carried out, the quality and medication safety and stability of a freeze-dried powder injection, a small-needle injection and a transfusion preparation are ensured, and application of the freeze-dried powder injection, the small-needle injection and the transfusion preparation in treatment of the symptoms of excessive phlegm and distressed cough of all respiratory system diseases including bronchitis is guaranteed.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Preparation method of fudosteine
The invention relates to a preparation method of fudosteine, which comprises the following steps: by using L-cysteine and trimethylene oxide as raw materials, heating under alkaline conditions to promote the completion of the reaction, adding 95% ethanol into the product to precipitate, and recrystallizing to obtain the fine product fudosteine, wherein the product yield is greater than or equal to 90%, the HPLC (high performance liquid chromatography) purity is greater than or equal to 99.5%, and the maximum single impurity content is less than or equal to 0.1%. The whole process is easy to control, simple to operate and suitable for industrial production, and is a synthesis route capable of satisfying the demands of the market for fudosteine active pharmaceutical ingredients.
Owner:迪嘉药业集团股份有限公司
Fudosteine solution preparation for atomization inhalation, and preparation method thereof
InactiveCN109925300AAvoid first pass effectAvoid destructionPowder deliveryOrganic active ingredientsFUDOSTEINEDisease
The invention provides a fudosteine solution preparation for atomization inhalation, and a preparation method thereof. A single dose of the fudosteine solution preparation for atomization inhalation comprises the following components: 20-120 mg of fudosteine or a salt and / or a hydrate thereof (based on free fudosteine), 0.5-2 mg of a metal complexing agent, an appropriate amount of a pH regulatorand water for injection. The prepared fudosteine solution preparation for atomization inhalation has the characteristics of high efficiency, low toxicity, good stability and high safety, and is used for treatment of sputum stickiness, expectoration difficulty and phlegm obstruction of trachea and other diseases caused by chronic bronchitis, bronchial asthma and other diseases.
Owner:SHANGHAI KAIBAO PHARMA +1
Method for preparing fudosteine molecularly imprinted polymer
InactiveCN102060958AEfficient enrichmentEfficient purificationOther chemical processesAlkali metal oxides/hydroxidesCross-linkFunctional monomer
The invention provides a method for preparing fudosteine molecularly imprinted polymer and belongs to the technical field of chemical engineering. The method comprises: dissolving fudosteine serving as template molecules, methacrylic acid serving as functional monomer and an ethylene glycol dimethacrylate serving as a cross-linking agent according to a molar ratio of 1:3-4:10, wherein a pore forming agent is mixed liquid of methanol and methylbenzene in a ratio of 1:1-3; adding azodiisobutyronitrile (AIBN) serving as an initiator; performing polymerization at 50 to 60 DEG C for 10 to 20 hours; milling the product, extracting with methanol-acetic acid solution for several times to remove template molecules, and drying at 60 DEG C and under vacuum to obtain fudosteine molecularly imprinted polymer. The fudosteine polymer prepared by the method can be used for detecting fudosteine residue in the environment or medicaments and the enrichment of trace amount of fudosteine, has the advantages of high speed, high flexibility, high accuracy, high yield, high repeatability and high sensitivity, and has a high application value and a bright market prospect.
Owner:TIANJIN POLYTECHNIC UNIV
Preparation method of Fudosteine
InactiveCN105968035AAvoid generatingQuality improvementOrganic chemistry methodsSulfide preparationFUDOSTEINEAlcohol
The invention discloses a preparation method of Fudosteine, and relates to a preparation method suitable for industrially producing Fudosteine. Water and ethyl alcohol are adopted as reaction solvent, amine substances serves as the catalyst, cooling and crystallization are conducted after reaction, and the product is obtained in the form of sedimentation. The whole process is easy to control, repeatability is high, the yield of Fudosteine is stabilized at 95%, the residual amount is controlled within 0.05%, and the preparation method completely conforms to the medicinal standard and is quite suitable for industrial production.
Owner:WEIHAI DISU PHARMA CO LTD
Sustained release composns preparation of Fudosteine and clarithromycin
InactiveCN1833652AEasy to useGood treatment effectPowder deliveryOrganic active ingredientsFUDOSTEINEDisease
A slowly-releasing composite medicine for treating the diseases in respiratory system is prepared from Fuduositan (15-30 Wt %), clarithromycin (10-40), and auxiliary (rest) including paralyser.
Owner:GUANGZHOU YOUYI PHARMA SCI & TECH DEV +1
Method for measuring fudosteine related substance by using amino column
The invention discloses a method for measuring a fudosteine related substance by using an amino column and belongs to the technical field of pharmaceutical analysis. A chromatographic column (25cm*4.6mm, 5 microns) taking amino bonded silica gel bonded silica gel as a filler serves as the chromatographic column used in the method, 0.05mol / L of potassium dihydrogen phosphate solution (the pH of phosphoric acid is adjusted to 4.5) and acetonitrile in the ratio of 35:65 serve as mobile phase, the detection wavelength is 210nm, the flow rate is 1.0mL / min, a high performance liquid chromatography condition is the column temperature of 30 DEG C, and the fudosteine related substance is measured. According to the related substance measuring method provided by the invention, more impurities can be measured, separation degree between a main peak and impurities is better, trace impurities in a sample can be more scientifically and accurately measured, the using safety and the efficiency of the sample are improved, and the method has advanced nature.
Owner:CHENGDU UNIV
Fudosteine inhalant composition
InactiveCN108078964AUniform contentIncrease the amount of deposition in the effective partPowder deliveryOrganic active ingredientsFUDOSTEINEMedicine
The invention belongs to the technical field of medicine, and relates to a fudosteine inhalant and a preparation method thereof. The fudosteine inhalant provided by the invention is prepared from 60 to 90 percent of fudosteine and 10 to 40 percent of lactose, wherein the percentage is the mass percentage; the particle diameter D90 of the fudosteine is preferably 2 mu m to 5 mu m. The invention provides a fudosteine dry powder inhalant with high bioavailability.
Owner:DISHA PHARMA GRP +1
Production technique of fudosteine
InactiveCN105622474AReduce Occupational HazardsEasy to useOrganic compound preparationSulfide preparationPotassium persulfateSolvent
The invention relates to a production technique of fudosteine. The production technique comprises the following steps: dissolving L-cysteine in purified water by stirring, adding potassium persulfate and propenol, and stirring to react for 5+ / -1 hours while controlling the temperature at 30+ / -2 DEG C; distilling under reduced pressure to remove the solvent; adding ethanol into the residues, stirring for 1.5 hours, and filtering to remove insoluble substances; concentrating the filtrate until a small amount of solid precipitates, and stopping concentrating; cooling, crystallizing by stirring at 30+ / -2 DEG C for 3+ / -1 hours until abundant white solid appears, and carrying out centrifugal filtration; washing the filter cake with anhydrous ethanol twice, and drying to obtain a fudosteine crude product; and refining and drying to obtain the fudosteine. By using ethanol-water as the solvent, the technique has the advantages of high use safety, low cost, mild reaction conditions and high atom economic benefit, and conforms to the requirements for green chemistry. The product yield is up to 95% or above, the content is up to 99.9%, and the maximum single impurity content is lower than 0.01%. Besides, the technique reduces the occupational hazards for employees, and is more beneficial to mass production.
Owner:ANHUI YOUCARE KAIYUE PHARMA
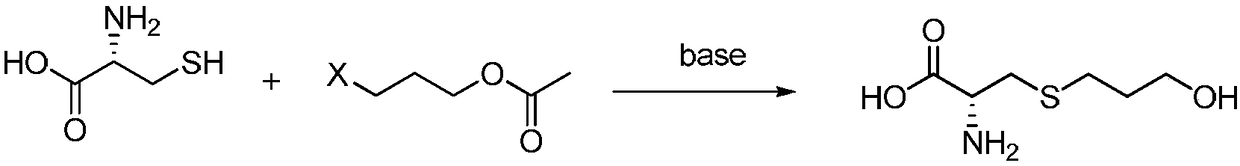
Preparation method of high-purity fudosteine
InactiveCN108586298AMild reaction conditionsImprove responseOrganic chemistry methodsSulfide preparationFUDOSTEINEPropionate
The invention discloses a preparation method of high-purity fudosteine, and belongs to the technical field of synthesis of pharmaceutical compounds. The preparation method is characterized in that with 3-halogenated propyl acetate or 3-halogenated propyl propionate and L-cysteine as starting materials, a fudosteine crude product is prepared through a reaction in an aqueous alkali solution, and high-purity fudosteine is obtained by refining the fudosteine crude product. The preparation method has the advantages that the reaction conditions are mild, the reaction process is simple, the yield ishigh, the cost is low, the requirement that the content of unknown impurities is lower than 0.1% can be met, and the preparation method is suitable for industrial application.
Owner:HENAN NORMAL UNIV
Composition containing ciclacillin or its derivatives and preparation thereof
InactiveCN101214245AHigh protection rateGood curative effectAntiinfectivesRespiratory disorderFUDOSTEINEActive component
The present invention provides a series of combination which contains ciclacillin or the derivative of the ciclacillin and is the combination essentially consisting of the ciclacillin or the derivative of the ciclacillin and an expectorant drug, wherein, the derivative of the ciclacillin is the officinal salt or officinal ester of the ciclacillin. The expectorant drug is any one of ambroxol, acetylcysteine, carbocisteine, erdosteine, fudosteine and lignum vitae glycerol ether. The unit medication dosage of the ciclacillin is 0.25g to 0.5g which is preferential (counted by active component), and the unit medication dosage of the expectorant drug is 0.01g to 2.0g. The combination is mainly used for remedying the respiratory tract infection with the symptom of expectoration.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Fudosteine oral liquid and preparation method thereof
ActiveCN112057418ADid not affect in vivo bioavailabilityReduce conversionAntibacterial agentsOrganic active ingredientsFUDOSTEINEChemical compound
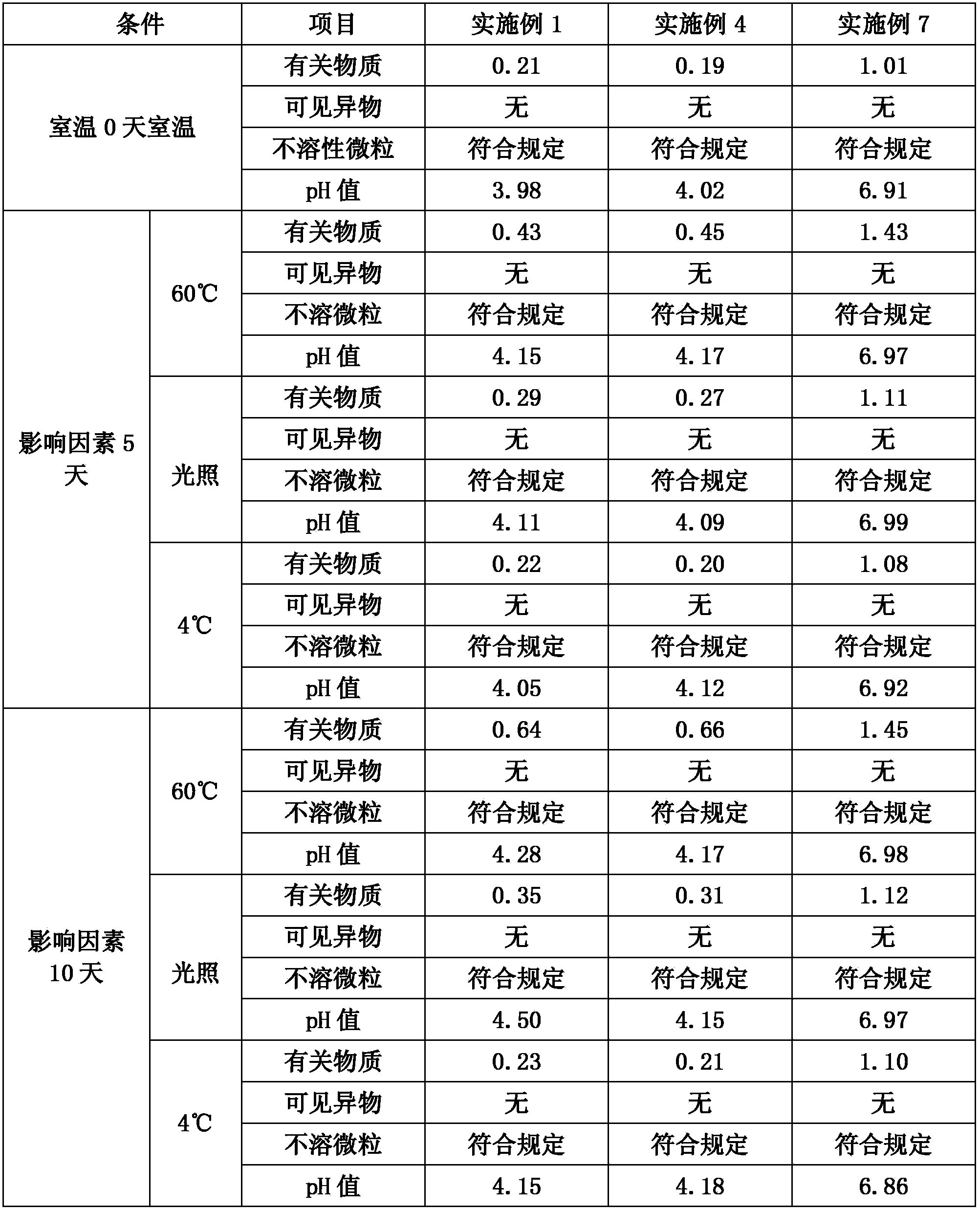
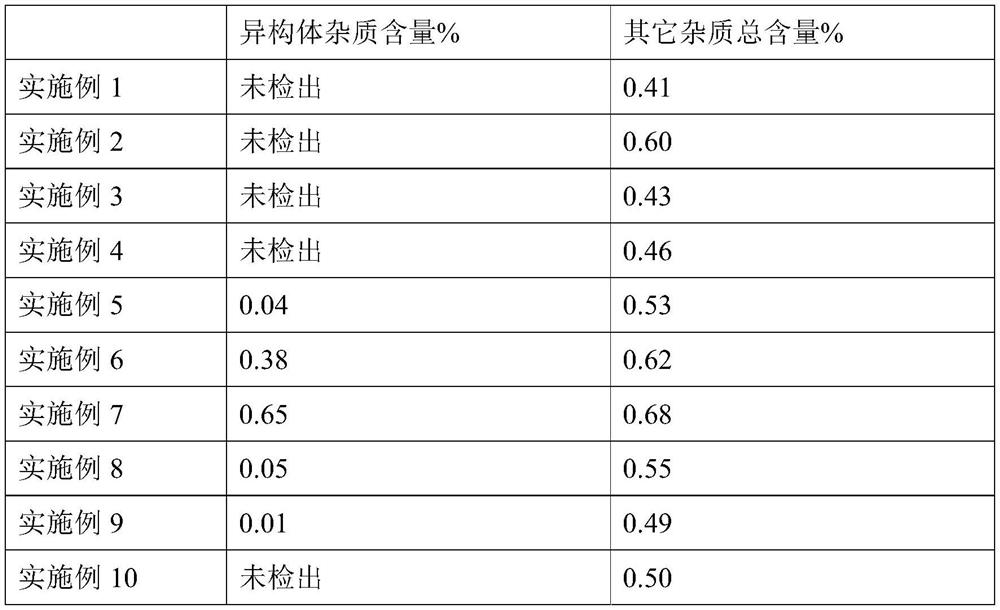
The invention relates to a fudosteine oral liquid and a preparation method thereof. The fudosteine oral liquid comprises fudosteine, a metal ion compound, an acid-base regulator and a solvent, whereinthe fudosteine and metal ions form a chelate through chelation. The preparation method comprises the following steps: (1) mixing the metal ion compound with part of the solvent, and stirring until the metal ion compound is dissolved; (2) mixing the solution obtained in the step (1) with the fudosteine, stirring until the solution is dissolved, and adding the acid-base regulator to regulate the pHvalue of the solution; and (3) carrying out chelation reaction on the solution obtained in the step (2), cooling, and adding the solvent to the target quantity, thereby obtaining the fudosteine oralliquid. The oral liquid has the advantages of favorable stability and lower isomer impurity content, and solves the problem that the isomer impurity content in the fudosteine oral liquid increases quickly as the time goes on in the prior art.
Owner:AC PHARMA CO LTD
Fudosteine oral solid composition
ActiveCN101152172BLow costSimple methodOrganic active ingredientsPharmaceutical non-active ingredientsFUDOSTEINEFiller Excipient
The invention discloses a stable fudosteine drug combination, containing carbohydrate and / or sugar alcohol selected from lactose and is used as the filler. The combination has good stability and is used to expel phlegm to stop cough.
Owner:AVENTIS PHARMA HAINAN
A kind of synthetic method of fudosteine
The invention discloses a synthesis method of fudosteine. The synthesis method comprises the following steps: in a glacial acetic acid-water system serving as a solvent, initiating a free radical reaction between L-cysteine and allyl alcohol which serve as starting raw materials at 40 DEG C by ultraviolet light; then, dropwise adding a certain amount of ethanol solution, cooling to 20 DEG C, and filtering to obtain a crude product of fudosteine; recrystallizing the crude product of fudosteine with 5-100% ethanol; filtering for the second time and drying to finally obtain high-purity fudosteine. The synthesis method of fudosteine is mild in synthesis condition and high in product yield which is higher than or equal to 92%; HPLC purity is higher than or equal to 99% and the maximum net contamination is less than or equal to 0.1%. The synthesis method of fudosteine can meet the demands of the market on fudosteine bulk drug.
Owner:YICHANG HEC CHANGJIANG PHARMA CO LTD
Fudosteine oxide impurity and preparation method thereof
ActiveCN103739526AQuality improvementSimple methodOrganic chemistryOrganic compound preparationFUDOSTEINEPropanoic acid
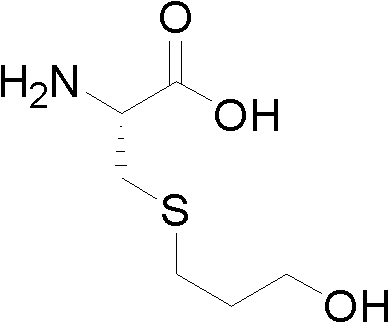
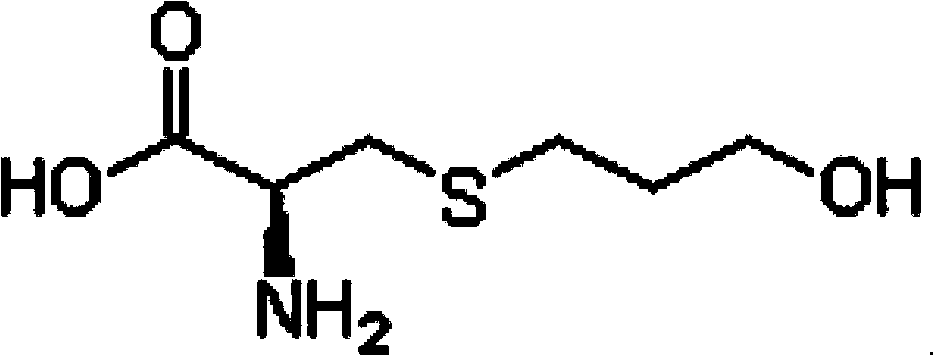
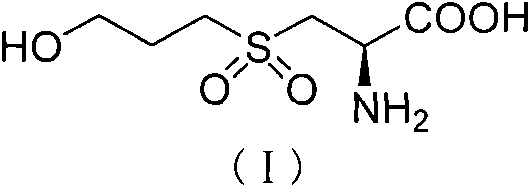
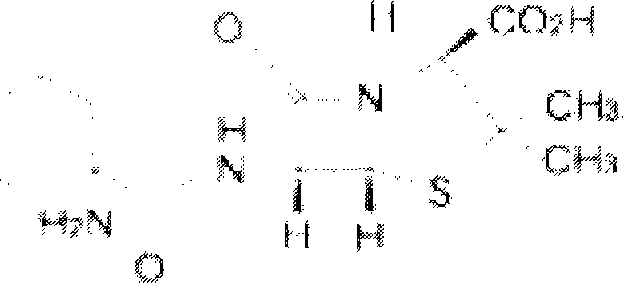
The invention relates to a Fudosteine oxide impurity (R)-2-amino-3-(3-hydroxypropyl sulfonyl) propionic acid (formula I shown in the specification) and a preparation method thereof. The pharmacological activity of Fudosteine (formula II shown in the specification) comes from closed sulfydryl in structure, which is metabolized into a free mercapto derivative in vivo to play a role. If sulphur atom is oxidized, normal metabolism can not be carried out in vivo to obtain the pharmacological activity. Therefore, existence of the impurity (formula I shown in the specification) has a strong impact on medicine quality and medication safety of Fudosteine and needs key control. The specific structure of the oxide impurity is as follows.
Owner:迪嘉药业集团股份有限公司
Fudosteine preparation method
The invention relates to a fudosteine preparation method. The method concretely comprises the following steps: dissolving metallic sodium in liquefied ammonia, adding cysteine, and adding bromopropyl alcohol in a dropwise manner; and evaporating ammonia, dissolving the residual solid in 1mol / L of hydrochloric acid, allowing the obtained solution to go through a Dowex50 (H+) column, eluting by using 1mol of ammonium hydroxide, and freeze-drying to obtain fudosteine. The fudosteine preparation method has the advantages of convenient and simple preparation, and low cost.
Owner:张云
Fudosteine synthesis method
The present invention mainly discloses a synthetic method of fudosteine, which adopts redox system to catalyze free radical reaction, uses L-cysteine as starting material, mixes it with appropriate amount of purified water, and mixes it with appropriate amount of purified water at a certain temperature Stir to dissolve at low temperature, add allyl alcohol, then add transition metal or its salts, gradually add water-soluble peroxide dropwise at a temperature of 0°C-50°C, keep warm for a period of time, filter to remove insoluble matter, and recover unreacted filtrate under reduced pressure Complete solvent and appropriate amount of water. After recovery, ethanol was added for crystallization. The obtained solid is re-crystallized with purified water and dried to obtain the finished product fudosteine (I). The synthesis method of the present invention has mild conditions, high product yield (≥90%), high content (≥99%), and low inorganic salt residue (≤0.01%), which is a very competitive synthetic route.
Owner:ZHEJIANG GUOBANG PHARMA
Fudosteine Solution Preparation for Aerosol Inhalation, and Preparation Method Therefor
PendingUS20200316003A1Reduce harmEasy to useOrganic active ingredientsPowder deliveryFUDOSTEINEMedicine
Disclosed are a fudosteine solution preparation for aerosol inhalation, and a preparation method therefor. The fudosteine solution preparation for aerosol inhalation comprises fudosteine, salts thereof and / or hydrates thereof; a metal complexing agent; and water for injection. The method for preparing the fudosteine solution preparation for aerosol inhalation includes the following steps: adding water for injection to a liquid formulation device and filling in nitrogen for protection, keeping the nitrogen in a liquid formulation tank at a positive pressure, and determining a residual oxygen content to be less than 2 mg / L; weighing the metal complexing agent, and stirring until the same dissolves; adding fudosteine, salts thereof and / or hydrates thereof, and stirring until the same dissolves; adding a pH adjusting agent, and determining the residual oxygen content to be less than 2 mg / L; supplementing with water for injection to a full amount, and stirring until evenly mixed; and carrying out fine filtration under sterile conditions, encapsulating and filling in nitrogen.
Owner:BEIJING INCREASE INNOVATIVE DRUG RESEARCH CO LTD
Fudosteine oral solid composition
ActiveCN101152172AImprove stabilityWon't change colorOrganic active ingredientsPharmaceutical non-active ingredientsFUDOSTEINEFiller Excipient
The invention discloses a stable fudosteine drug combination, containing carbohydrate and / or sugar alcohol selected from lactose and is used as the filler. The combination has good stability and is used to expel phlegm to stop cough.
Owner:AVENTIS PHARMA HAINAN
Preparation method of fudosteine crystals
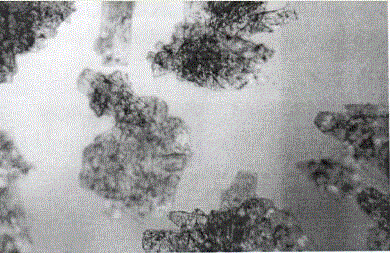
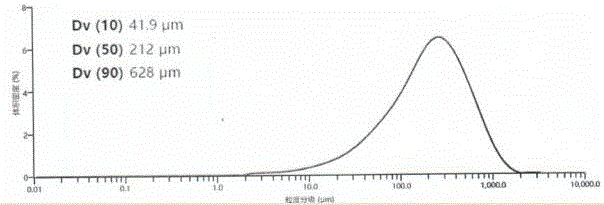
ActiveCN106432021AUniform particle size distributionSuitable for industrial productionOrganic compound preparationOrganic chemistry methodsFUDOSTEINEOrganic solvent
The invention relates to a preparation method of fudosteine crystals, and belongs to the technical field of crystals. The technical scheme of the invention is that the preparation method of the fudosteine crystals of which the main grain sizes are ranged from 180 to 220 microns comprises the following steps: 1, adding the fudosteine to water, wherein the solution solid-liquid ratio is 0.5g / ml to 1.0g / ml, and continuously stirring and dissolving for 30 to 60 minutes at the temperature of 40 to 50 DEG C; 2, carrying out filtration and decolorization; moving a filtrate into a crystallizer, lowering the temperature to 10-20 DEG C, wherein the cooling rate is 5-20 DEG C / h, adding crystal seeds to the crystallizer, cultivating the crystals for 1-2 hours; then carrying out fed-batch of organic solvents of which the weights are 4 to 10 times of that of water in initial solutions to the crystallizer, wherein the fed-batch rate is 2-10 mL / min, and cultivating the crystals for 1-3 hours; 3, filtering, and washing filter cakes by using washing solvents, finally drying products, thus obtaining the fudosteine products with uniform size distribution.
Owner:迪嘉药业集团股份有限公司
Medicinal composition
Medicinal compositions characterized by containing fudosteine and an antipyretic / analgesic. These medicinal compositions are medicinal compositions to be used for common cold, etc. which have improved sputum-removal and antiinflammatory effects.
Owner:SS PHARMA CO LTD
Azitromycin double salts and their preparing method and use
InactiveCN1775794AAcid stableGood water solubilityOrganic active ingredientsSugar derivativesAzithromycinFUDOSTEINE
The invention relates to an azithromycin fudosteine compound salt. Its feature is that it combines azithromycin and fudosteine, which is double to azithromycin. And its molecular formula is that C38H72N2O12.2C6H13NO3S.nH2O, n=0,1,2,3,4,5. Its advantages are that it has good water solubility and is uneasy to absorption of moisture; the aqueous solution pH value acidity is close to human-body. Using the azithromycin fudosteine compound salt as the main medicine can make various pre oral and injection preparation. The preparations are mainly used to treat abundant expectoration type infection of the respiratory.
Owner:西安新安医药科技有限公司
Combination containing clacillin or derivative and preparing method thereof
The invention relates to a series of combined substances which contain cyclacillin or derivative of the cyclacillin, in particular to a combined substance where the cyclacillin or the derivative of the cyclacillin and an expectorant are combined. Wherein, the derivative of the cyclacillin is medicine salt or medicine ester of the cyclacillin; and the expectorant is any of ambroxol, acetylcysteine, carbocisteine, erdosteine, fudosteine and guaiacum glycerol ether. The drug dosage per unit of the cyclacillin is in preference to 0.25g to 0.5g ( the content is calculated according to active components). The drug dosage per unit of the expectorant is 0.01 to 2.0g. The combined substance is mainly used to treat respiratory tract infection with expectoration symptom.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com