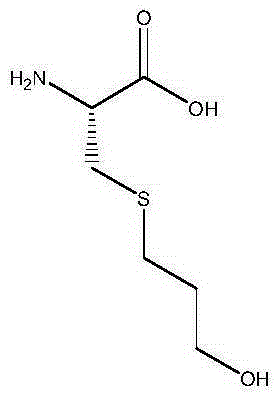
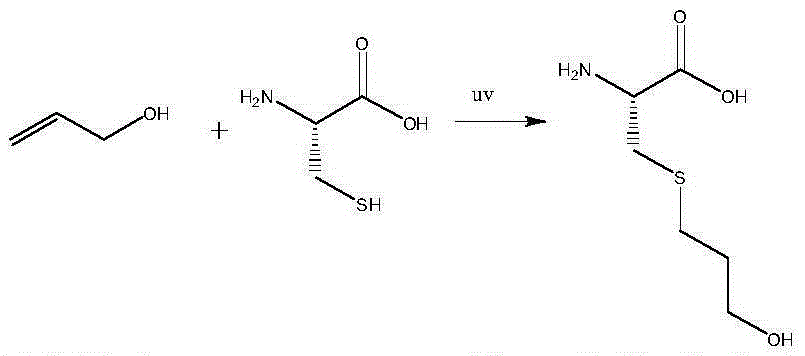
A kind of synthetic method of fudosteine
A synthesis method and the technology of fodosteine, which are applied in sulfide preparation, organic chemistry and other directions, can solve the problems of low product purity and low yield, and achieve the effects of high product yield and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Take 20g of L-cysteine, put it into a 1000mL four-necked bottle, add 140g of purified water, add dropwise 35mL of glacial acetic acid, mix and dissolve at 40°C, and add dropwise 15g of allyl alcohol. React under 365nm ultraviolet light for 8 hours, add 800mL of 95% ethanol dropwise, cool to 20°C, keep warm for 2h, filter, wash the filter cake with 50mL of 95% ethanol, dry to obtain 29.1g of crude fudosteine, and then use 450mL of 15% The ethanol was heated to 40°C, stirred and dissolved, cooled to 20°C after 2 hours, filtered, the filter cake was washed with 50mL of 95% ethanol, and dried to obtain 28.2g of fudosteine, with a yield of 94%, a purity of 99.83% by HPLC, and a maximum purity of 0.06 %.
Embodiment 2
[0018] Take 20g of L-cysteine, put it into a 1000mL four-necked bottle, add 140g of purified water, add dropwise 55mL of glacial acetic acid, mix and dissolve at 40°C, and add dropwise 20g of allyl alcohol. React under 210nm ultraviolet light irradiation for 6h, add 700mL85% ethanol dropwise, cool down to 20°C, keep warm for 2h, filter, wash the filter cake with 50mL85% ethanol, dry to obtain 28.8g crude fudosteine, and then use 550mL25% The ethanol was heated to 40°C, stirred and dissolved, cooled to 20°C after 2 hours, filtered, the filter cake was washed with 50mL of 85% ethanol, and dried to obtain 27.6g of fudosteine, with a yield of 92%, a purity of 99.75% by HPLC, and a maximum purity of 0.04 %.
Embodiment 3
[0020] Take 20g of L-cysteine, put it into a 1000mL four-necked bottle, add 140g of purified water, add dropwise 30mL of glacial acetic acid, mix and dissolve at 40°C, and add dropwise 17g of allyl alcohol. React under 280nm ultraviolet light irradiation for 6h, add dropwise 900mL of absolute ethanol, cool to 20°C, keep warm for 2h, filter, wash the filter cake with 50mL of absolute ethanol, dry to obtain 29.3g of crude product fudosteine, and then use 550mL of 25% ethanol was heated to 40°C, stirred and dissolved, cooled to 20°C after 2 hours, filtered, the filter cake was washed with 50mL of absolute ethanol, and dried to obtain 28.5g of fudosteine, the yield was 95%, and the purity by HPLC was 99.59%. Simplex 0.07%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com