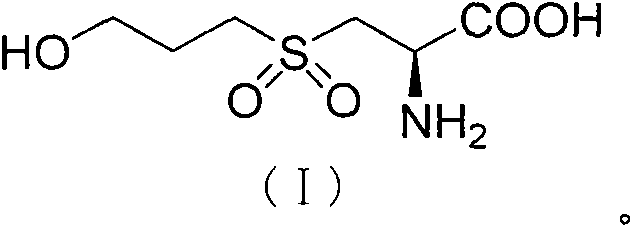
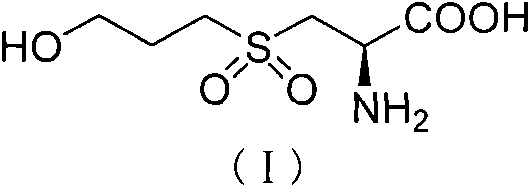
Fudosteine oxide impurity and preparation method thereof
A technology for oxidizing impurities and fodosteine, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems affecting the quality and safety of fodosteine drugs, difficult to obtain high purity, etc. The effect of high product purity and improved drug quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Synthesis of (R)-2-(tert-butoxycarbonylamino)-3-(3 hydroxypropylthio)propionic acid
[0038] Dissolve 10.84g fudosteine and 2.66g sodium hydroxide in 100ml water and 40ml tert-butanol, add 13.85g di-tert-butyl dicarbonate (Boc 2 O), stirred reaction, overnight at room temperature. The reaction solution was adjusted to pH 1-2 with potassium bisulfate, then extracted with 2*40mL ethyl acetate, dried over anhydrous magnesium sulfate, and concentrated to obtain (R)-2-(tert-butoxycarbonylamino)-3-(3-hydroxy Propylthio)propionic acid.
Embodiment 2
[0039] Embodiment 2: Synthesis of (R)-2-(tert-butoxycarbonylamino)-3-(3 hydroxypropylthio)propionic acid benzyl ester
[0040] Dissolve (R)-2-(tert-butoxycarbonylamino)-3-(3-hydroxypropylthio)propionic acid obtained in the previous step in 50ml of methanol, adjust the pH to 7 with potassium bicarbonate, spin off the solvent and dry in vacuo . After drying, dissolve in 55ml of DMF, add 11.79g of benzyl bromide, react at room temperature for 5h, dilute with 150mL of water, and extract with 2*40mL of ethyl acetate. The organic layer was washed with 50 mL of water, dried over anhydrous magnesium sulfate, and concentrated to obtain 18.63 g of benzyl (R)-2-(tert-butoxycarbonylamino)-3-(3-hydroxypropylthio)propionate. The two-step yield is 83.2%.
Embodiment 3
[0041] Example 3: Synthesis of (R)-benzyl 2-(tert-butoxycarbonylamino)-3-(3 hydroxypropylsulfonyl)propionate.
[0042] Dissolve 17.15g of benzyl (R)-2-(tert-butoxycarbonylamino)-3-(3-hydroxypropylthio)propionate in 20ml of methanol, and slowly add 42.36g of potassium hydrogen persulfate under ice cooling Composite salt solution (content 42%, dissolved in 20ml of water), removed from the ice room temperature and reacted overnight. The methanol was removed under reduced pressure, the residue was dissolved in 60 mL of water, extracted with 3*30 mL of ethyl acetate, the organic layers were combined, washed with 30 mL of water, dried over anhydrous magnesium sulfate to obtain (R)-2-(tert-butoxycarbonylamino 13.8 g of benzyl )-3-(3 hydroxypropylsulfonyl)propionate, yield 75%, purity 98.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com