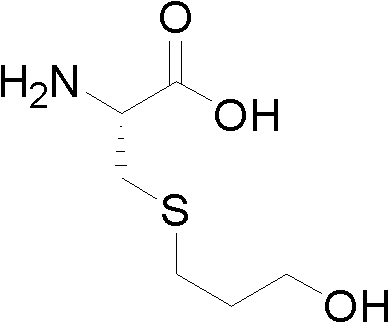
Method for preparing high-purity Fudosteine
A technology for fodosteine and a purification method, which is applied in the field of purification for preparing high-purity fodosteine, can solve problems such as unmarked fodosteine purity, and achieves a safe and controllable quality, mild conditions and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]Suspend 200g of L-cysteine in 150ml of water, adjust the pH of the suspension to 9 with 2N sodium hydroxide solution, add 400ml of ethanol and 252g of 3-bromo-1-propanol to the suspension, stir overnight, and the reaction is complete Finally, adjust the pH to 5 with 10% hydrochloric acid solution, evaporate the solvent under reduced pressure, wash the residue repeatedly with 1500ml of acetone, and mash to obtain a powder; the obtained powder is subjected to ion exchange chromatography and treated with 15000ml of 2N ammonia water as the eluent, and the eluate is collected After concentrating under reduced pressure, the residue is fudosteine crude product;
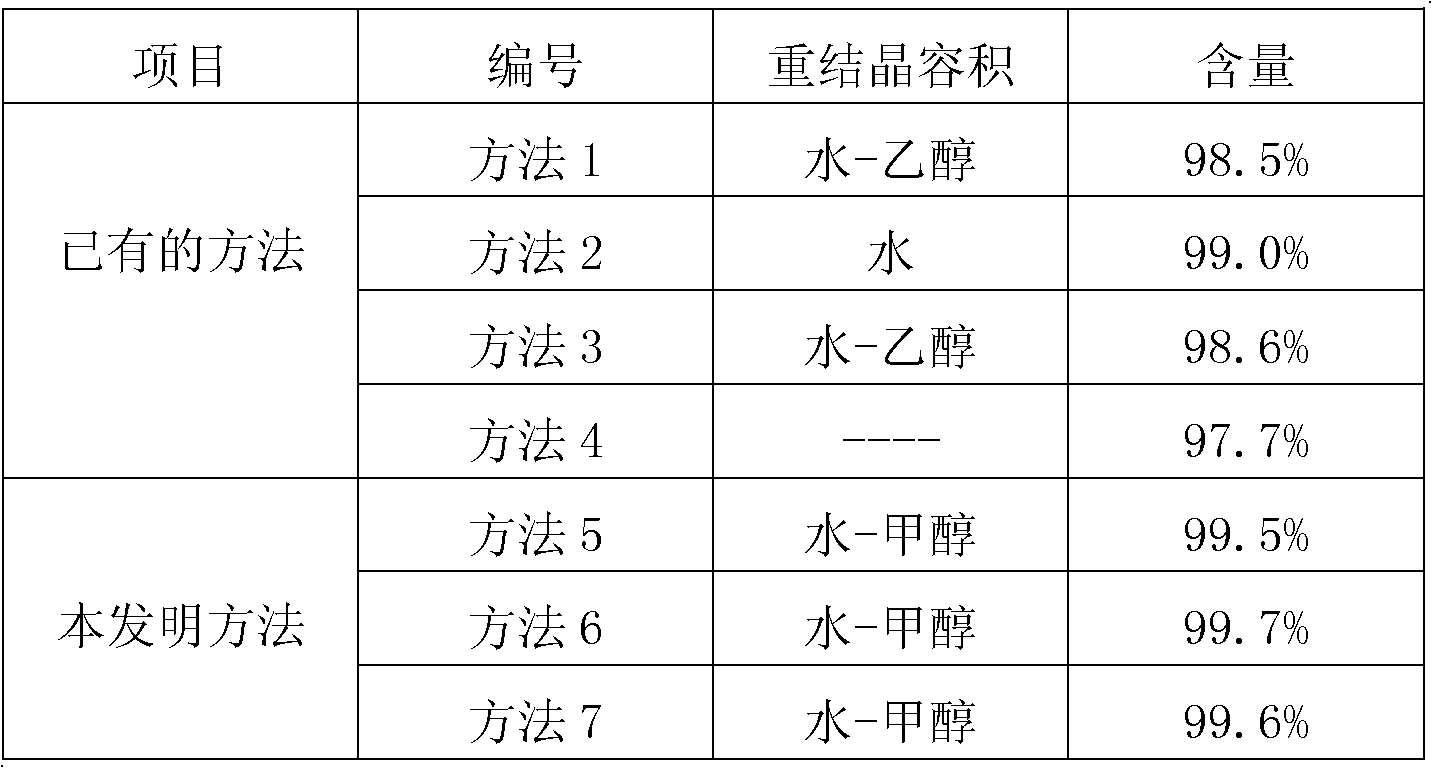
[0043] Take 100g of crude product of fudosteine and add 50 times of water to dissolve at 90°C, add 0.03 times of the amount of water used for adsorption and decolorization of activated carbon, filter, add 5g of methanol, crystallize at -20°C, filter and dry to obtain 82g of pure product of fordosteine , the HPLC ...
Embodiment 2
[0045] Suspend 200g of L-cysteine in a small amount of 150ml of water, adjust the pH of the suspension to 9 with 2N sodium hydroxide solution, add 400ml of ethanol and 252g of 3-bromo-1-propanol to the suspension, stir overnight, and react Adjust the pH to 5 with 10% hydrochloric acid solution, evaporate the solvent under reduced pressure, wash the residue repeatedly with 1500ml of acetone and crush to obtain powder; Concentrate under reduced pressure after liquid to obtain residue as crude product of fudosteine;
[0046] Take 100 g of the crude product of fudosteine and add 500 times of water to dissolve at 20°C, add 0.01 times of the amount of water used for adsorption and decolorization of activated carbon, filter, add 5000ml of methanol, crystallize at 10°C, filter and dry to obtain 79g of pure fordosteine, The HPLC purity of fudosteine obtained was 99.7%.
Embodiment 3
[0048] Suspend 200g of L-cysteine in a small amount of 150ml of water, adjust the pH of the suspension to 9 with 2N sodium hydroxide solution, add 400ml of ethanol and 252g of 3-bromo-1-propanol to the suspension, stir overnight, and react Adjust the pH to 5 with 10% hydrochloric acid solution, evaporate the solvent under reduced pressure, wash the residue repeatedly with 1500ml of acetone and crush to obtain powder; Concentrate under reduced pressure after liquid to obtain residue as crude product of fudosteine;
[0049] Take 100 g of crude product of fudosteine and add 300 times of water to dissolve at 50 ° C, add 0.04 times of the amount of water used for adsorption and decolorization of activated carbon, filter, add 900 g of methanol, crystallize at 0 ° C, filter and dry to obtain 93 g of pure product of fudosteine, The HPLC purity of fudosteine obtained was 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com