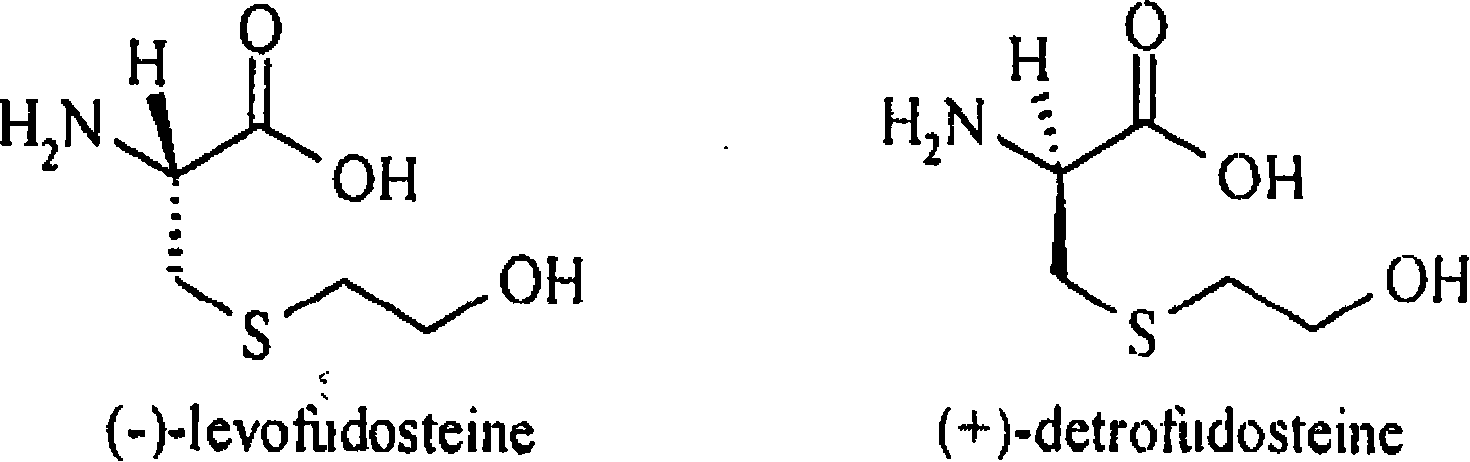Method for decomposing chiral mobile phase additive RP-HPLC of fudosteine enantiomer
A mobile phase additive, RP-HPLC technology, applied in the field of analytical chemistry, can solve the problems of cumbersome and time-consuming derivatization reaction, expensive CSP column, etc., and achieve the effect of quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Instruments and Conditions 1
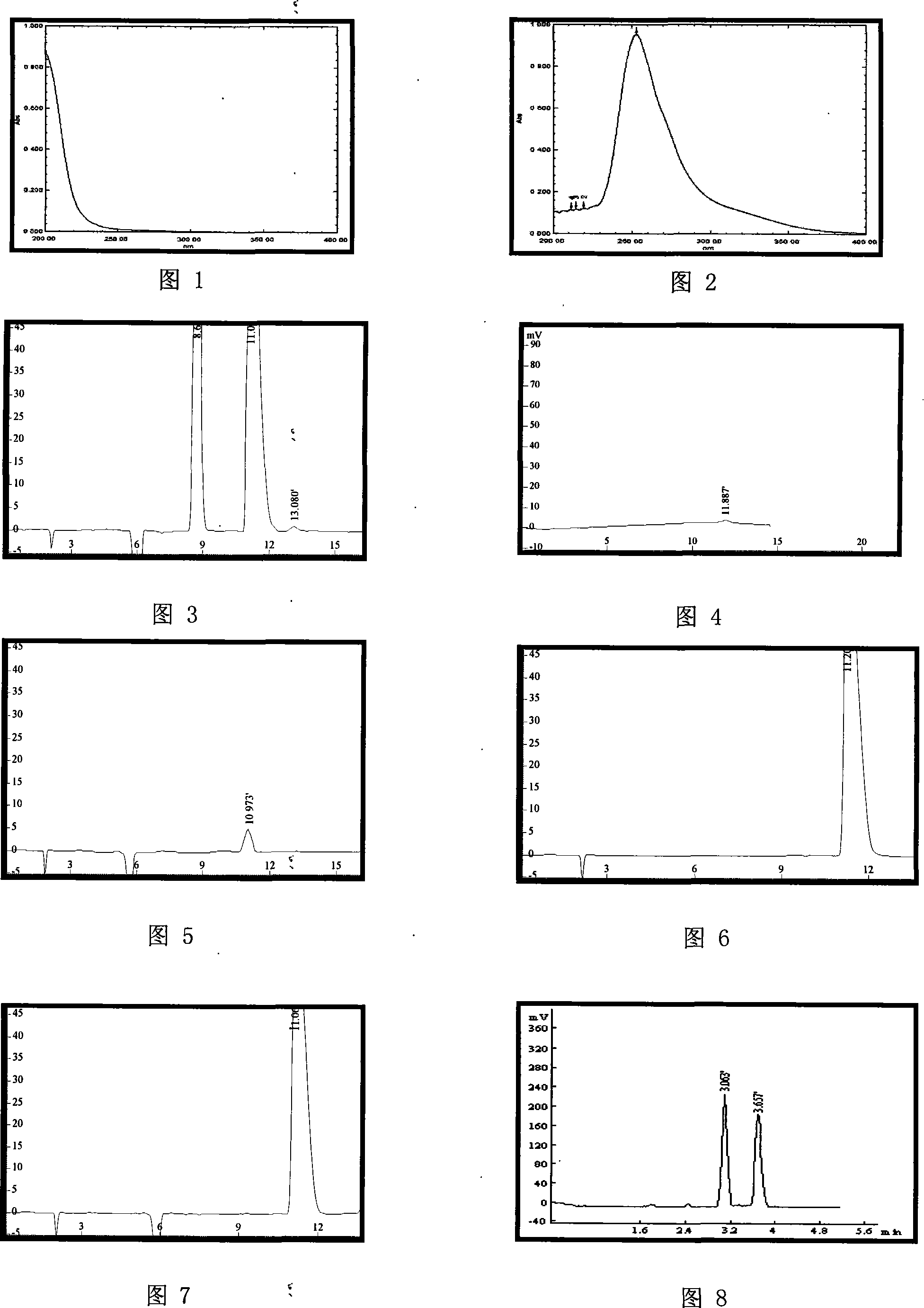
[0026] Shimadzu 10Avp high performance liquid chromatograph, octadecylsilane bonded silica gel as filler, L-phenylalanine solution (take L-phenylalanine 1.0g, copper sulfate 1.3g, add water 1000ml to dissolve) as Mobile phase, UV detection wavelength is 275nm, detection limit 1ug / ml, see Figure 4.
[0027] Implementation steps
[0028] Take an appropriate amount of fudosteine racemate, add mobile phase solution to make a solution containing 5mg per 1ml. Take 20 μl and inject it into a liquid chromatograph, the peak order is D-(+)-enantiomer first, L-(-)-enantiomer last, and the separation degree between enantiomers is greater than 1.5. The number of theoretical plates calculated based on the L-(-)-enantiomer peak should not be less than 1000. The area ratio of the front to back peaks of the enantiomers in the recorded chromatogram should be 0.5-2. Generally, the retention time of the D-(+)-enantiomer is about 9 minutes, and the reten...
Embodiment 2
[0030] Get fudosteine appropriate amount, add mobile phase solution and make the solution that contains fudosteine 5mg in every 1ml, as need testing solution; Under the chromatographic condition of embodiment 1, measure need testing solution 20 μ l injection liquid phase Chromatograph, the retention time of the main peak in the chromatogram of the test solution should be consistent with the retention time of the L-(-)-enantiomer peak of racemic fudosteine, see Figure 3, 5-6.
Embodiment 3
[0032] Get an appropriate amount of fudosteine tablet powder, add mobile phase solution to make a solution containing 5mg of fudosteine in every 1ml, as the test solution. Under the chromatographic condition of embodiment 1, measure need testing solution 20 μ l and inject liquid chromatograph, the main peak retention time in need testing solution chromatogram should be with the L-(-)-enantiomer of racemic fudosteine The peak retention time is consistent, see Figure 3, 5-7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com