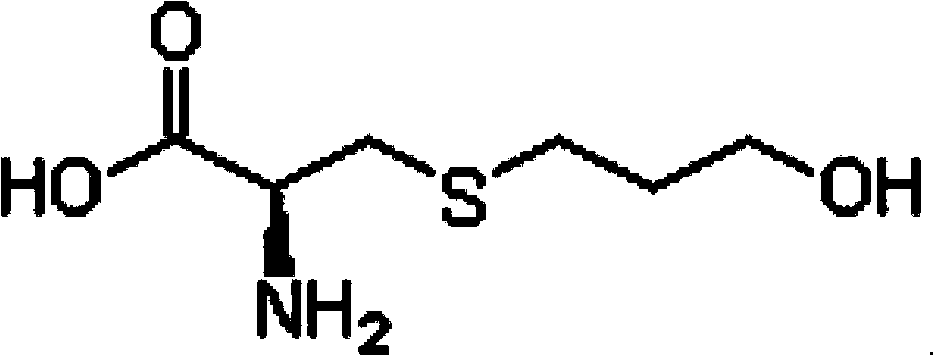
Fudosteine injection and preparation method thereof
A technology for fudosteine and injection, applied in the field of pharmaceutical preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
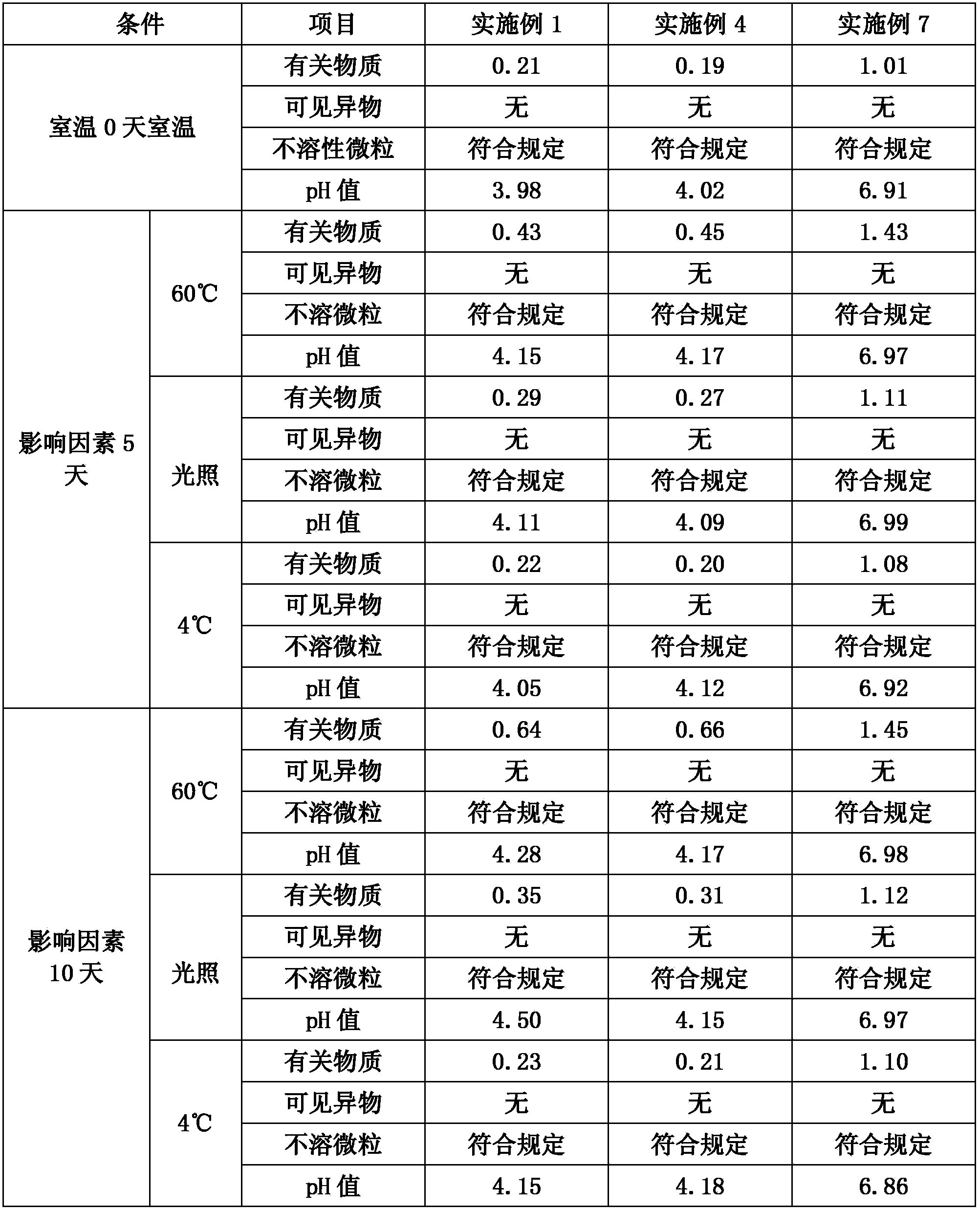
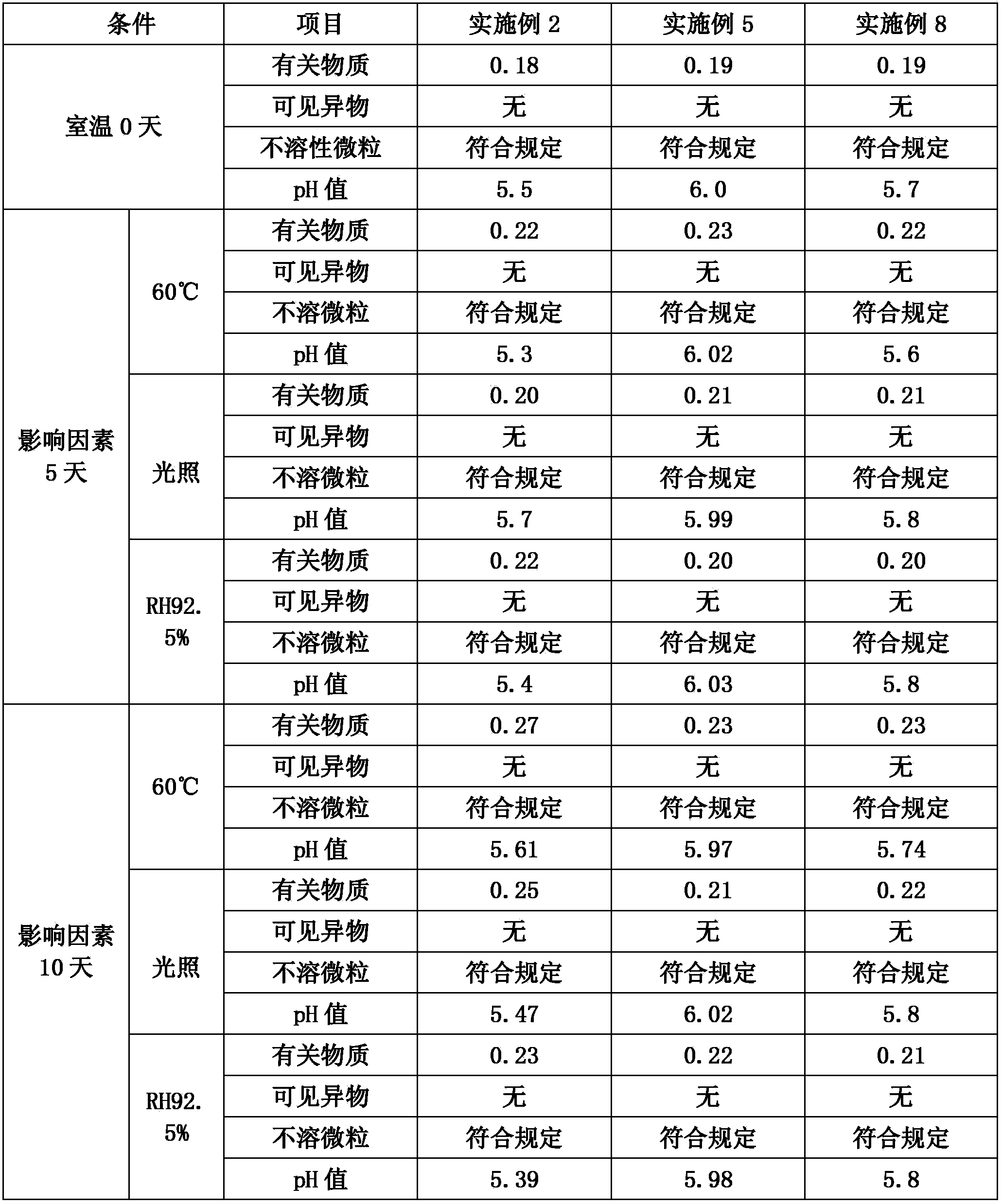
Embodiment 1
[0031] 1000 sticks
[0032] prescription:
[0033] Fordosteine
200g
0.2g
sodium citrate
0.6g
Add water for injection to
2000ml
[0034] Process:
[0035] Weigh the prescribed amount of fudosteine, citric acid and sodium citrate into a beaker, add 1500ml of water for injection, stir at room temperature to dissolve, add 1mol / L hydrochloric acid or 1mol / L sodium hydroxide to adjust the pH to 3-6. Add 0.1% activated carbon, stir at room temperature for 20 minutes, filter to remove carbon, measure the content of intermediates, add water for injection to make up for fine filtration with a 0.22μm filter membrane, fill with 2ml each, and sterilize at 121°C for 15 minutes to obtain Defudos Tan injection.
Embodiment 2
[0036] 1000 sticks
[0037] prescription:
[0038] Fordosteine
400g
400g
Add water for injection to
1000ml
[0039] Process:
[0040] First add 800ml of water for injection into the container, add fudosteine and mannitol and stir to dissolve at room temperature for about 30 minutes, add 0.2% activated carbon, stir at room temperature for 20 minutes, decarburize, use 0.22μm microporous membrane to filter bacteria, measure the middle Add water for injection to make up, subpackage by 1ml per bottle, pre-freeze for 2 hours, dry under reduced pressure for 12 hours under freezing, and dry for 3 hours after the sample temperature reaches room temperature to obtain a white loose block. Seal the seal to get fodosteine freeze-dried powder injection.
Embodiment 3
[0041] 50 bottles
[0042] prescription:
[0043] Fordosteine
10g
110g
2g
Add water for injection to
12500ml
[0044] Process:
[0045] Add 10000ml of water for injection in the container, add fudosteine, potassium chloride and disodium hydrogen phosphate and stir to dissolve, stir and dissolve at room temperature for about 30 minutes, add 1mol / L phosphoric acid to adjust the pH to 3-6, add 0.2% activated carbon, and keep at room temperature Stir for 20 minutes, decarburize, measure the content of intermediates, add water for injection to make up, use 0.22μm microporous membrane filter, pack in 250ml per bottle, and sterilize at 115°C for 15 minutes to obtain Defodosteine Injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com