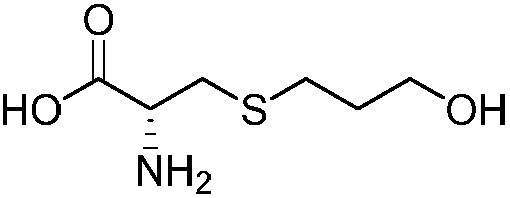
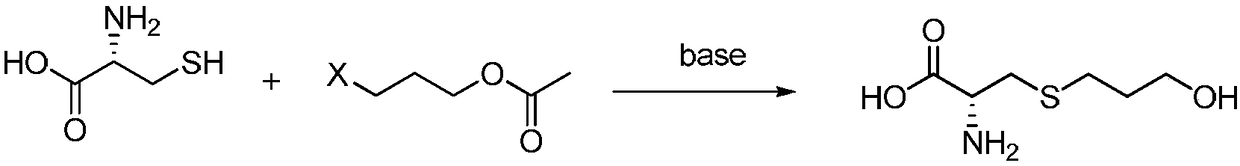
Preparation method of high-purity fudosteine
A high-purity, water-soluble technology, applied in the field of preparation of high-purity fudosteine, can solve the problems of unreachable unknown impurities, difficult source of starting materials, high unknown impurities, etc., achieve low cost, mild reaction conditions, The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] In the there-necked flask, add 30mL (0.22mol) of triethylamine, take 100g of distilled water, after dissolving, the temperature drops to -20°C, then add 13.3g (0.11mol) of L-cysteine, stir to dissolve, slowly drop Solubilize the ethanol (10g) solution that has 20g (0.11mol) of 3-bromopropyl acetate, after the HPLC monitoring reaction finishes, extract three times with 67g n-heptane, adjust pH to 5-6 with nitric acid in aqueous phase, concentrate, Obtain crude fodosteine.
[0024] In a one-mouth bottle, add the crude product of fudosteine mentioned above, heat in an oil bath until dissolved, add 135g of acetone to reflux for 1h, heat-preserve and suction filter, reflux the filtrate for 1h, cool down to crystallize, and suction filter, and the obtained filter cake is refluxed with 90g of ethanol for beating for 1h. After suction filtration and drying of the filter cake, 19.2 g of refined fudosteine was obtained, with a molar yield of 97.5%, an HPLC purity of 99.9%, an...
Embodiment 2
[0026] In the there-necked flask, add 64g (0.6mol) of sodium carbonate, take 290g of distilled water, after dissolving, the temperature drops to 30°C, then add 14.5g (0.12mol) of L-cysteine, stir to dissolve, and the temperature rises to 100 ℃, slowly dropwise add the methanol (150g) solution that is dissolved in 50g (0.37mol) of 3-chloropropyl acetate, HPLC monitors that the reaction finishes, after the reaction finishes, extract three times with 290g n-hexane, and adjust the pH of the aqueous phase with hydrochloric acid to 5-6, concentrated to obtain the crude product of fudosteine.
[0027] In a one-mouth bottle, add the above-mentioned fudosteine crude product, heat in an oil bath until dissolved, add 460 g of ethanol to reflux for 1 h, heat-preserve and suction filter, reflux the filtrate for 1 h, cool down to crystallize, and suction filter, and obtain a filter cake with 230 g of ethanol to reflux for beating for 1 h. After filtration, the filter cake was dried to obt...
Embodiment 3
[0029] In the there-necked flask, add 25g (0.44mol) of potassium hydroxide, take 500g of distilled water, after dissolving, the temperature drops to 0°C, then add 26.6g (0.22mol) of L-cysteine, stir to dissolve, slowly add Dissolve the acetone (25g) solution of 50g (0.22mol) of 3-iodopropyl acetate, HPLC monitors the end of the reaction, after the end of the reaction, extract three times with 266g cyclohexane, adjust the pH of the aqueous phase to 5-6 with formic acid, Concentrate to obtain the crude product of fudosteine.
[0030] In a single-necked bottle, add the crude product of fudosteine mentioned above, heat in an oil bath until dissolved, add 720 g of methanol to reflux for 1 hour, heat-preserve and suction filter, reflux the filtrate for 1 hour, cool down to crystallize, and suction filter, and the obtained filter cake is refluxed with 270 g of ethanol for beating for 1 hour. After suction filtration and drying of the filter cake, 38.9 g of refined fudosteine was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com