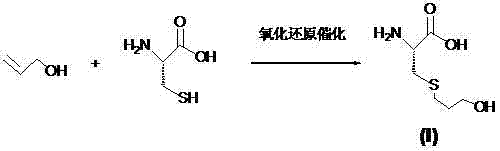
Fudosteine synthesis method
A synthesis method and cysteine technology are applied in the preparation of thioether, organic chemistry and other directions, and can solve the problems of difficult removal of inorganic salts, low product purity and low yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 1. Take 30g (0.25mol) of L-cysteine, put it into a 1000ml four-necked flask, add 330g of purified water, mix, stir and dissolve at 25°C, add 21.6g (0.37mol) of propenyl alcohol, and then add After adding 0.03g of elemental iron, slowly add 3g of hydrogen peroxide with a concentration of 1% dropwise at a temperature of 0°C-5°C, dropwise for 5min, after dropping, keep warm for 30min at a temperature of 0°C-5°C, After heat preservation, heat up to 70°C, filter, heat up the filtrate, and recover the solvent under reduced pressure at a vacuum degree above 0.08Mpa. After solids are precipitated, stop the recovery, heat up to complete dissolution, and then slowly add 20 times the weight ratio dropwise while cooling. When cooled to 20°C, stir for 1 hour, shake off the filter, dry the filter cake, add 0.5 times the weight ratio of purified water for recrystallization, heat up to reflux, keep warm for 30min, and then slowly drop while cooling Add 5 times the weight rat...
Embodiment 2
[0022] Example 2 1. Take 30g (0.25mol) of L-cysteine, put it into a 1000ml four-necked flask, add 330g of purified water, mix, stir and dissolve at 25°C, add 15.1g (0.26mol) of allyl alcohol, and then add 6g of elemental aluminum, after adding, slowly add 10gH at a concentration of 1% at a temperature of 10°C-15°C 2 O 2 , add dropwise for 10 minutes, after dropping, keep warm for 30 minutes at a temperature of 10°C-15°C, finish keeping warm, heat up to 70°C, filter, heat up the filtrate, recover the solvent under reduced pressure at a vacuum degree of 0.08Mpa or more, and wait for solids to precipitate out. Stop recovery, heat up to complete dissolution, then slowly add anhydrous ethanol dropwise with a weight ratio of 20 times while cooling, when cooled to 20°C, stir for another hour, shake off the filter, dry the filter cake, and add 0.5 times the weight ratio Recrystallize in purified water, heat up to reflux, keep warm for 30 minutes, then slowly add 5 times the weight ...
Embodiment 3
[0023] Example 3 1. Take 30g (0.25mol) of L-cysteine, put it into a 1000ml four-necked flask, add 330g of purified water, mix, stir and dissolve at 25°C, add 43.14g (0.74mol) of propenyl alcohol, and then add 0.6g of elemental zinc, after adding, slowly add 10g of tert-butyl hydroperoxide with a concentration of 5% dropwise at a temperature of 10°C-15°C, dropwise for 10min, after dropping, at a temperature of 10°C-15°C Insulate for 1 hour, heat up to 70°C, filter, heat up the filtrate, recover the solvent under reduced pressure at a vacuum of 0.08Mpa, stop the recovery after solids are precipitated, heat up to complete dissolution, then slowly add dropwise while cooling Anhydrous ethanol with a weight ratio of 30 times is cooled to 30°C, then stirred for 1 hour, filtered, and the filter cake is dried, then recrystallized with purified water with a weight ratio of 3 times, heated to reflux, kept for 30 minutes, and then cooled , while slowly adding 15 times the weight ratio o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com