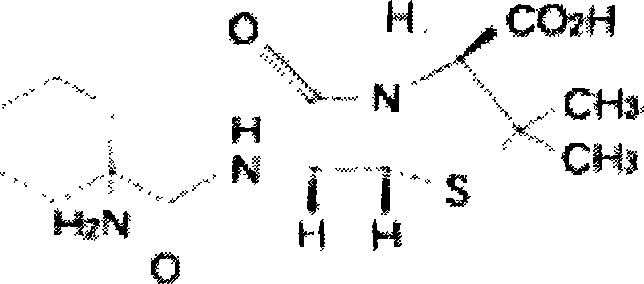
Composition containing ciclacillin or its derivatives and preparation thereof
A technology of cyclohexycillin and composition, applied in the field of medicine, can solve the problems of poor antibacterial activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 cyclohexicillin and ambroxol hydrochloride tablet
[0025] prescription:
[0026] components
Dosage
Cyclohexacillin Sodium
CMS-Na
Micropowder silica gel
Co-made
250g (calculated based on the active ingredient cyclohexicillin)
30g
90g
5g
5g
1000 pieces
[0027] Preparation:
[0028] Pass cyclohexicillin, ambroxol hydrochloride, and microcrystalline cellulose through a 80-mesh sieve, mix well, and set aside; another appropriate amount of 50% ethanol solution is used as a binder soft material, granulated with a 24-mesh sieve, and dried at 50°C , 20-mesh sieve, add micropowder silica gel, CMS-Na, mix evenly, and press a suitable die to form a tablet.
[0029] If the above-mentioned tablet is coated, a coated tablet can be obtained, which can be a film-coated tablet, an enteric-coated tablet, and the ...
Embodiment 2
[0030] Example 2: Cyclohexicillin and Acetylcysteine Granules
[0031] prescription:
[0032] components
Dosage
Cyclohexacillin pivaloyloxymethyl ester
Co-made
250g (calculated based on the active ingredient cyclohexicillin)
300g
1430g
20g
1000 bags
[0033] Preparation:
[0034] Mix cyclohexicillin and part of sucrose powder evenly, use purified water to make soft material, granulate with 18 mesh, dry, granulate with 16 mesh, and set aside; in addition, acetylcysteine and sucrose crystals are sieved with 16 mesh respectively particles to obtain crystal particles. Mix the above three kinds of granules, add sweet orange powder essence and pack them separately to get the product.
Embodiment 3
[0035] Example 3: Cyclohexicillin and Carbocisteine Chewable Tablets
[0036] prescription:
[0037] components
Dosage
Cyclohexicillin
Ansai K
Co-made
250g (calculated based on the active ingredient cyclohexicillin)
500g
650g
25g
25g
1000 pieces
[0038] Preparation:
[0039] Grind cyclohexicillin, carbocisteine, and xylitol and mix them evenly, use 2% starch slurry to make soft material, granulate with a 16-mesh sieve, dry, granulate with a 12-mesh sieve, add sweet orange powder essence and acesulfame potassium , fully mix evenly, adjust the weight of the content, and press it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com