Intramolecular adjuvant-containing recombinant gene for preventing and treating helicobacter pylori, protein and biological product
A technology of Helicobacter pylori and amino acids, applied in the direction of medical preparations containing active ingredients, gene therapy, bacterial antigen components, etc., can solve the problems of restriction and toxicity application, and achieve the effect of preventing damage and protecting biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1: Design and construction of multi-target fusion polypeptide
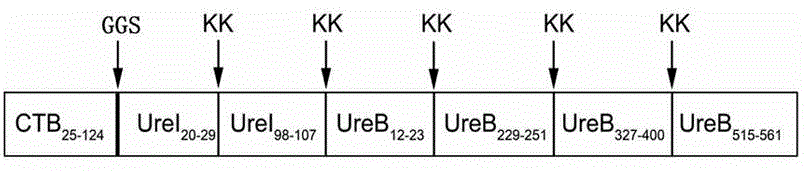
[0065] Such as figure 1 Shown is a schematic diagram of the construction of a multi-target fusion polypeptide BIB vaccine: BIB described in the present invention is to add a section of intramolecular adjuvant CTB that removes the signal peptide to the N segment of the multi-target fusion polypeptide IB, forming such as SEQ ID NO: The amino acid sequence shown in 2. The DNA sequence corresponding to the above amino acid sequence is the nucleotide sequence shown in SEQ ID NO:1. The protein sequence length of this BIB is 289aa, and protein molecular weight is 32.82 kDa, and protein isoelectric point is 9.05 pI, and its amino acid sequence is as shown in SEQ ID NO: 2, and the protein sequence length of CTB is 100aa, and protein molecular weight is 11.32 kDa, and protein The isoelectric point is 7.97 pI.
Embodiment 2
[0066] Embodiment 2: the preparation of rBIB engineering bacteria
[0067] The length of the BIB gene sequence is 876bp, which was synthesized by Beijing Jinweizhi; then the fusion gene BIB was constructed on the pET28a (+) vector, and the restriction endonuclease site was Bam H I and Hind III; after that, transform into Escherichia coli BL21 (DE3), screen positive clones, and construct vaccine engineering bacteria BIB.
Embodiment 3
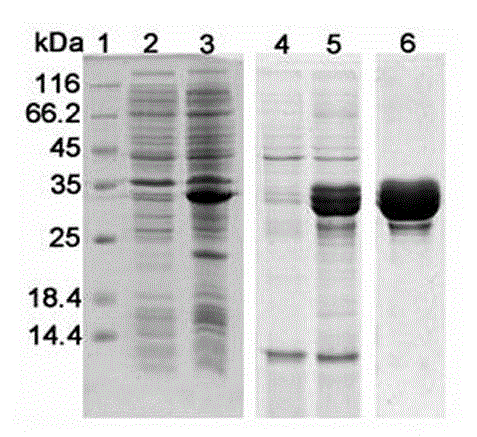
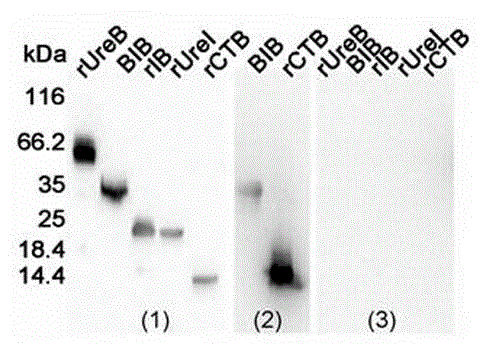
[0068] Embodiment 3: Preparation of rBIB protein
[0069] 3.1 Fermentation of BIB engineering bacteria
[0070] The improved TB medium was used as the fermentation medium, after high temperature and high pressure sterilization, inoculated with 10% inoculum, cultured at 37°C for 8-10 h, after that, added lactose with a final concentration of 5 mM for 10 h, and controlled the pH at 7.2±0.3, adjust the speed to control the dissolved oxygen above 30%. Finally, the yield of BIB cells was between 30-45 g / L; the protein expression was between 25%-35%.
[0071] 3.2 Preparation of BIB inclusion bodies
[0072] Take 1 kg / time of BIB engineering bacteria collected after fermentation and centrifugation, resuspend in ultrasonic lysis buffer at a ratio of 1:10 (W / V), and stir with a magnetic stirrer to mix evenly. Place the beaker of the uniformly mixed bacterial solution in ice cubes, pre-cool to 4°C, and use a sonicator at 250 W, on for 2 s, off for 2 s, and for 40 min. Take a small a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Electric point | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com