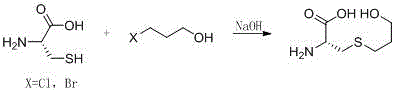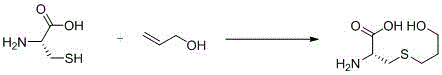Preparation method of fudosteine
A technology of fodosteine and cysteine, which is applied in the field of medicine, can solve the problems of short reaction time, easy residual heavy metals, low purity and the like, and achieves the effects of mild reaction conditions, high atom utilization rate and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] At room temperature, dissolve 20g of L-cysteine in 200mL of water, add 7.2g of sodium hydroxide, stir for 1 hour, cool down to 0-10°C, and drop 12.5g of oxetane into the reaction system , after dropping, rise to room temperature and stir for 2 hours, heat up to 40-50°C and stir for 6 hours, cool to room temperature, adjust pH to 6 with 6mol / L hydrochloric acid, distill under reduced pressure to 1 / 10 of the original volume, and heat up to 80°C Filtrate hot, slowly add 600mL of 95% ethanol to the filtrate, cool down to 0-10°C and stir for 2 hours after addition, filter, rinse the filter cake with 30mL of ethanol, blow dry at 40-50°C until constant weight to obtain 29.0g of crude product. Add the obtained crude product into a 1000ml four-neck flask, add 570mL of 20% ethanol aqueous solution, heat up to 50-60°C, after all dissolve, gradually cool down to room temperature and stir for 1h, cool down to 0-10°C, stir for 2h, filter, 40 Blast drying at -50°C to constant weight...
Embodiment 2
[0024] At room temperature, dissolve 20g of L-cysteine in 200mL of water, add 4.0g of sodium hydroxide, stir for 1 hour, cool down to 0-10°C, and drop 19.2g of oxetane into the reaction system , dropwise rise to room temperature and stir for 2 hours, heat up to 40-50°C and stir for 8 hours, cool to room temperature, adjust pH to 6 with 6mol / L hydrochloric acid, distill under reduced pressure to 1 / 10 of the original volume, heat up to 80°C After hot filtration, slowly add 600mL of 95% ethanol to the filtrate, cool down to 0-10°C and stir for 2 hours after addition, filter, rinse the filter cake with 30mL of ethanol, and blow dry at 40-50°C until constant weight to obtain 27.9g of crude product. Add the obtained crude product into a 1000ml four-neck flask, add 550mL of 15% ethanol aqueous solution, heat up to 50-60°C, after all dissolve, gradually cool down to room temperature and stir for 1h, cool down to 0-10°C, stir for 2h, filter, 40 Blast drying at -50°C to constant weigh...
Embodiment 3
[0026] At room temperature, dissolve 20g of L-cysteine in 100mL of water, add 9.0g of sodium hydroxide, stir for 1 hour, cool down to 0-10°C, and drop 10.5g of oxetane into the reaction system , dropwise rise to room temperature and stir for 2 hours, heat up to 40-50°C and stir for 5 hours, cool to room temperature, adjust pH to 6 with 6mol / L hydrochloric acid, distill under reduced pressure to 1 / 6 of the original volume, heat up to 85°C After hot filtration, slowly add 300mL of 95% ethanol to the filtrate, cool down to 0-10°C and stir for 2 hours after addition, filter, rinse the filter cake with 30mL of ethanol, blow dry at 40-50°C until constant weight to obtain 28.4g of crude product. Add the obtained crude product into a 1000ml four-neck flask, add 560mL of 30% ethanol aqueous solution, heat up to 50-60°C, after all dissolve, gradually cool down to room temperature and stir for 1h, cool down to 0-10°C, stir for 2h, filter, 40 Blast drying at -50°C to constant weight yie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com