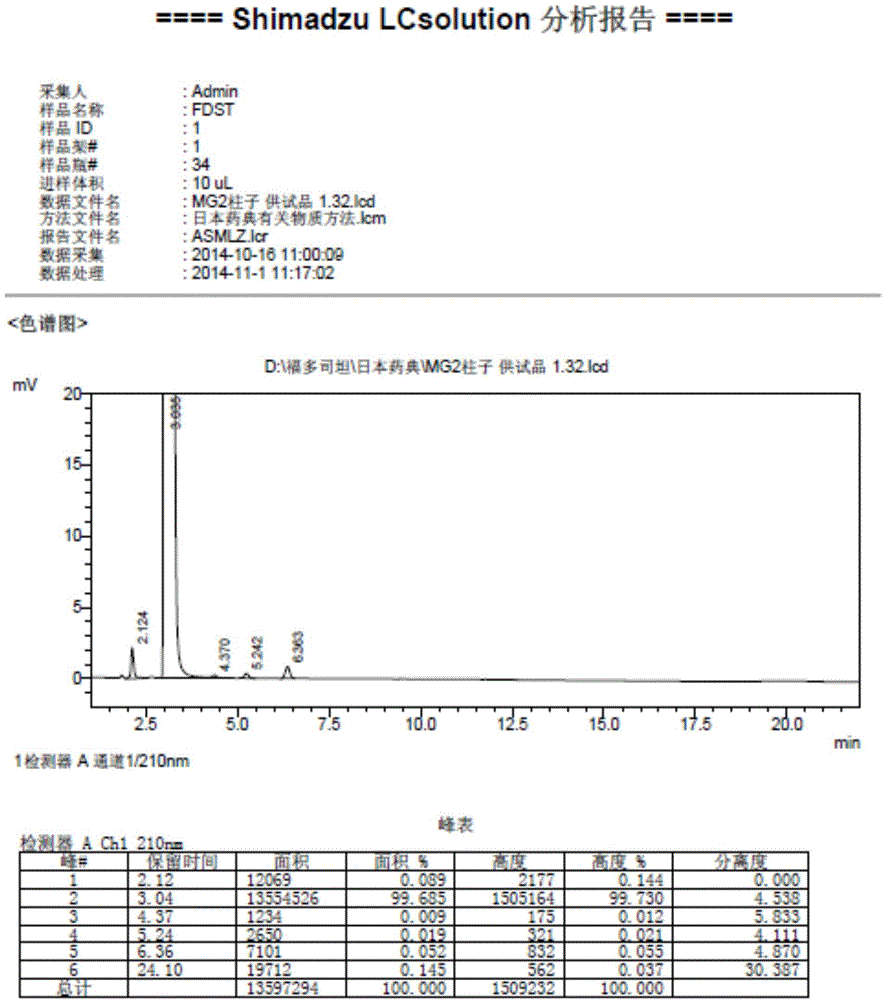
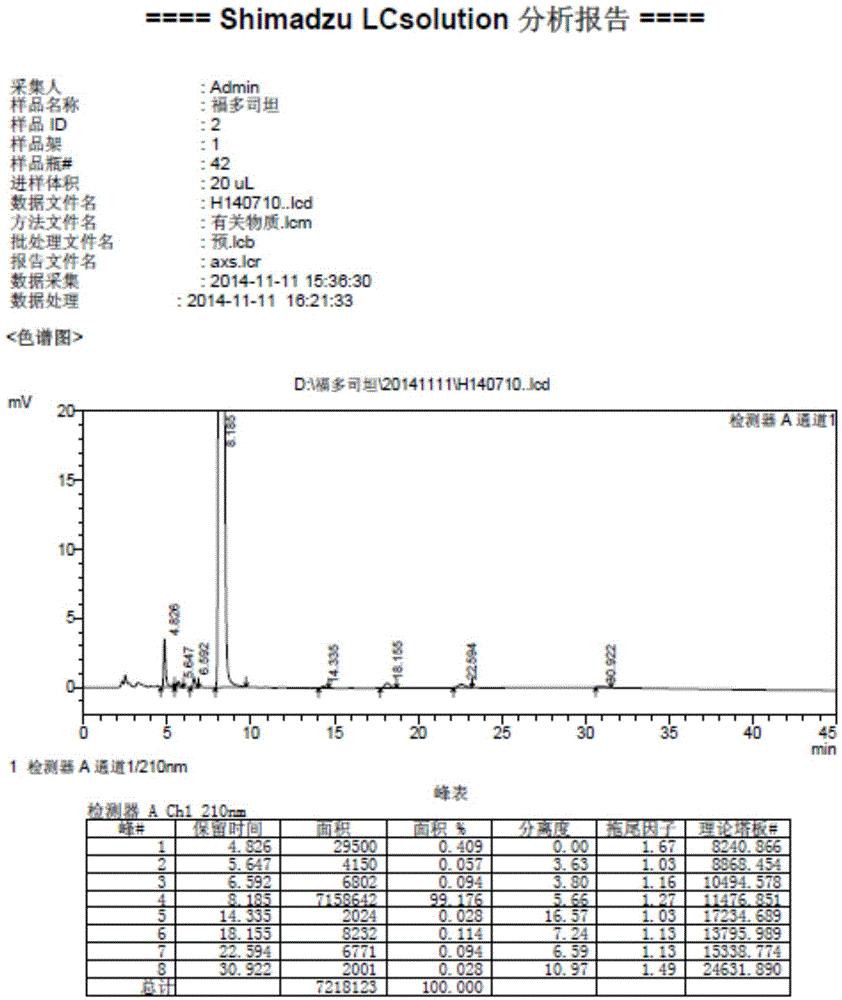
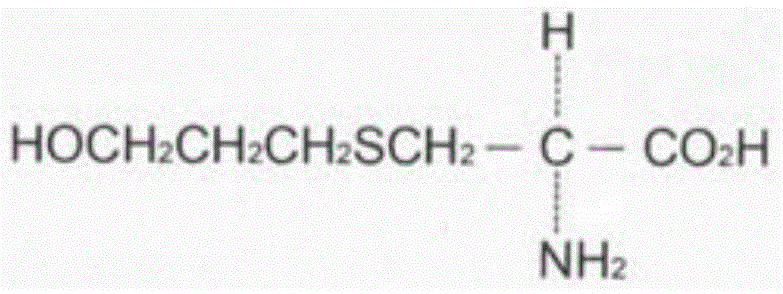Method for measuring fudosteine related substance by using amino column
A technology for related substances and determination methods, which is applied in the field of determination of related substances of fudosteine by using an amino column, can solve the problems of difficult impurities to be separated, poor repeatability of measurement results, large damage to fillers, etc., and achieve good durability and accuracy And the effect of high sensitivity and quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. Optimization of chromatographic conditions:
[0023] We compared (1) the selection of acetonitrile ratio by experiment: use 0.05mol / L KH 2 PO 4 -Acetonitrile (50:50), (40:60), (35:65), other conditions remain unchanged, the test is carried out, the result shows that the retention time of the concentration of acetonitrile of 65% is moderate, the degree of separation of impurities is good, and the proportion of acetonitrile of 65% is selected for use; (2) Selection of buffer salt concentration: other conditions remain unchanged for KH 2 PO 4 The concentration of the salt is selected, and a total of 5 salt concentrations of 0.1mol / L, 0.05mol / L, 0.02mol / L, 0.01mol / L, and 0.005mol / L are screened, and the result is that the buffer salt concentration has an impact on the peak shape. The higher the salt concentration, the sharper the peak, when the buffer salt concentration is 0.1mol / L KH 2 PO 4 When the baseline is uneven and the pressure fluctuates greatly, the salt c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com