Preparation method of hydroxychloroquine sulfate
A technology of hydroxychloroquine sulfate and hydroxychloroquine hydrochloride, applied in organic chemistry and other directions, can solve problems such as unfavorable industrial production, large amount of solvent, low solubility, etc., to avoid the risk of toxic substances, good product crystallinity, and reduced labor intensity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthesis of embodiment 1 hydroxychloroquine
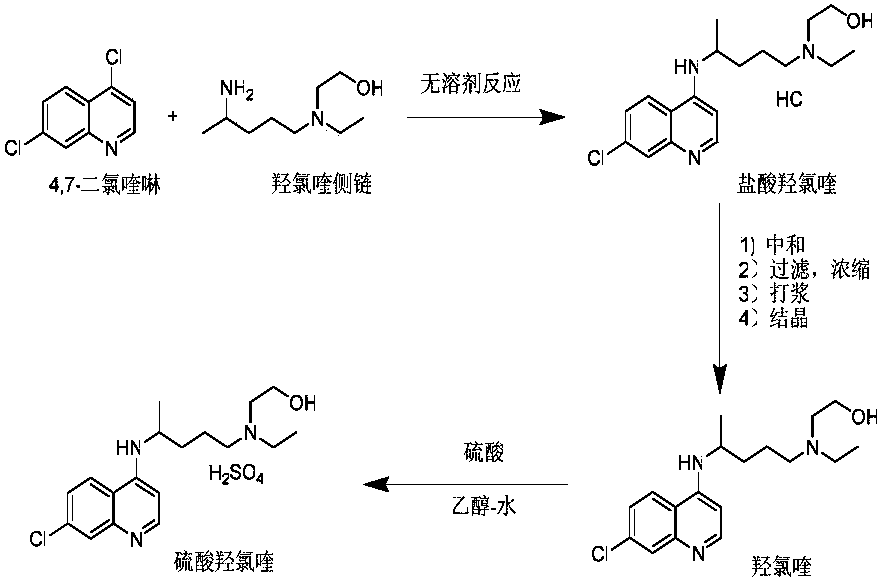
[0029] In a 500mL four-necked flask, add 4,7-dichloroquinoline: 100g (0.51mol) and hydroxychloroquine side chain: 89g (0.51mol), heat up to 110°C for 1 hour, then heat up to 120°C for 24 hours , point the board to monitor until 4,7-dichloroquinoline basically disappears, cool slightly, add anhydrous methanol (600mL), stir to dissolve, add 1.0eq sodium methoxide (27.5g) in batches, stir for 2h, filter to remove insoluble matter, the filtrate Concentrate under reduced pressure to dryness, then add methyl acetate (300 mL) to make a slurry, and filter to obtain 169 g of crude hydroxychloroquine.
[0030] Add the above-mentioned crude hydroxychloroquine to a mixed solvent of methanol-methyl acetate (1:4, v / v, 600mL), heat to dissolve, then naturally cool to room temperature, stir and crystallize for 5h, filter, and the filter cake is moistened with methyl acetate. Wash, dry at 50 DEG C with forced air to constant weight, an...
Embodiment 2
[0031] The synthesis of embodiment 2 hydroxychloroquine
[0032] In a 500mL four-necked flask, add 4,7-dichloroquinoline: 100g (0.51mol) and hydroxychloroquine side chain: 92g (0.53mol), heat up to 110°C for 1 hour, then heat up to 125°C for 20 hours , point the plate to monitor until 4,7-dichloroquinoline disappears substantially, cool to room temperature, add ethanol (800mL) to dissolve, add 1.1eq sodium ethoxide (38.15g) in batches, stir for 1-2h, filter to remove insoluble matter, and the filtrate reduces Concentrate under pressure to dryness, add isopropyl acetate to make a slurry, and filter to obtain crude hydroxychloroquine (168 g).
[0033] Add the above crude hydroxychloroquine to a mixed solvent of ethanol-ethyl acetate (1:5, v / v, 800mL), heat to dissolve, then naturally cool to room temperature, stir and crystallize for 8h, filter, and filter the cake with isopropyl acetate After rinsing, air-drying at 50°C to constant weight, about 146 g of refined hydroxychloroq...
Embodiment 3
[0034] The synthesis of embodiment 3 hydroxychloroquine
[0035] In a 500mL four-necked flask, add 4,7-dichloroquinoline: 100g (0.51mol) and hydroxychloroquine side chain: 95g (0.55mol), heat up to 110°C for 1 hour, then heat up to 130°C for 18 hours , point the plate to monitor until 4,7-dichloroquinoline disappears, cool to room temperature, add absolute ethanol to dissolve (1000mL), add 1.05eq of potassium tert-butoxide (59.9g) in batches, stir for 1-2h, filter to remove insoluble The filtrate was concentrated to dryness under reduced pressure, and ethyl acetate was added to make a slurry, and filtered to obtain 167.9 g of crude hydroxychloroquine.
[0036] Add the above crude hydroxychloroquine to a mixed solvent of ethanol-ethyl acetate (1:6, v / v, 1000mL), heat to dissolve, then naturally cool to room temperature, stir and crystallize for 6h, filter, and the filter cake is moistened with ethyl acetate. Wash, dry at 50 DEG C with forced air to constant weight, and obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com