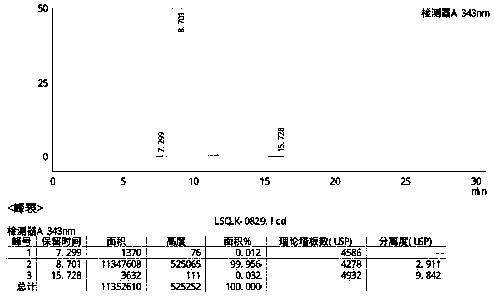
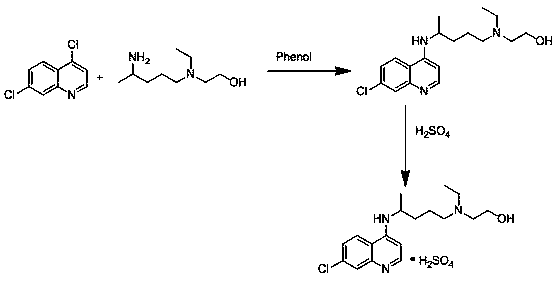
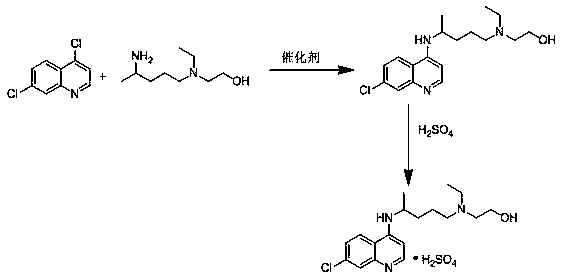
Novel preparation method of hydroxychloroquine sulfate
A technology of hydroxychloroquine sulfate and hydroxychloroquine, which is applied in the field of medicine and chemical industry, can solve the problems affecting the purification of hydroxychloroquine free base, high difficulty in operation, and difficult removal, so as to reduce production cost and pollution control cost, and the post-processing is simple and avoids The effect of impurity inclusion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation of potassium fluoride supported on alumina
[0037] Dissolve 10g of anhydrous potassium fluoride and 30g of 200-mesh alumina powder in 100ml of water, stir ultrasonically at 50 Hz for 45 minutes, evaporate the water under reduced pressure at 55°C to obtain a solid powder, dry it in vacuum at 120°C for 8 hours, take it out, and grind it into a powder After that, 40 g of catalyst was obtained.
Embodiment 2
[0038] Example 2: Preparation of tetrabutylammonium fluoride supported on alumina
[0039] Dissolve 40g of tetrabutylammonium fluoride and 40g of 200-mesh alumina powder in 100ml of water, stir ultrasonically at 50 Hz for 45 minutes, evaporate the water to dryness under reduced pressure at 55°C to obtain a solid powder, dry it in vacuum at 130°C for 8 hours, remove it, and grind it into powder to obtain 80 g of catalyst.
Embodiment 3
[0040] Embodiment 3: Preparation of cesium fluoride supported on alumina
[0041] Dissolve 10g of cesium fluoride and 20g of 200-mesh alumina powder in 100ml of water, stir ultrasonically at 50 Hz for 1 hour, evaporate the water under reduced pressure at 55°C to obtain a solid powder, dry it in vacuum at 100°C for 4 hours, take it out, and grind it into a powder , to obtain 30g catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com