Industrial preparation method of 4,7-dichloroquinoline
A technology of dichloroquinoline and chloroquinoline, which is applied in the field of preparation of pharmaceutical intermediate 4,7-dichloroquinoline, and achieves the effects of high yield, high product purity and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
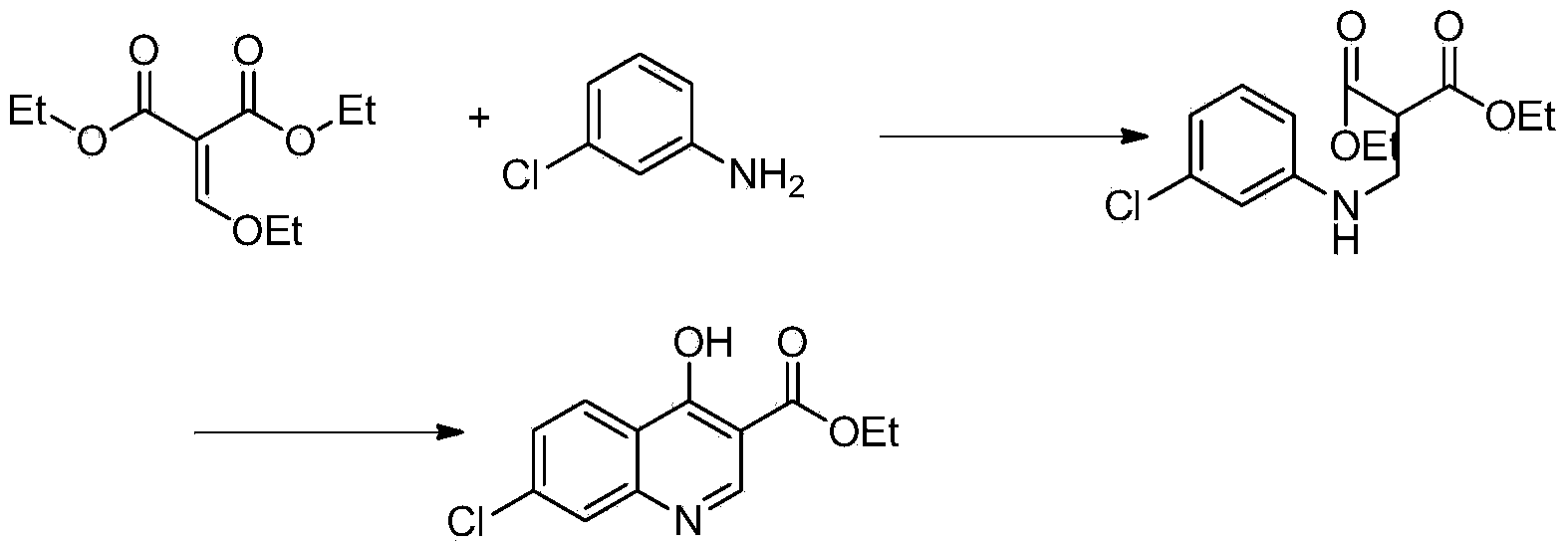
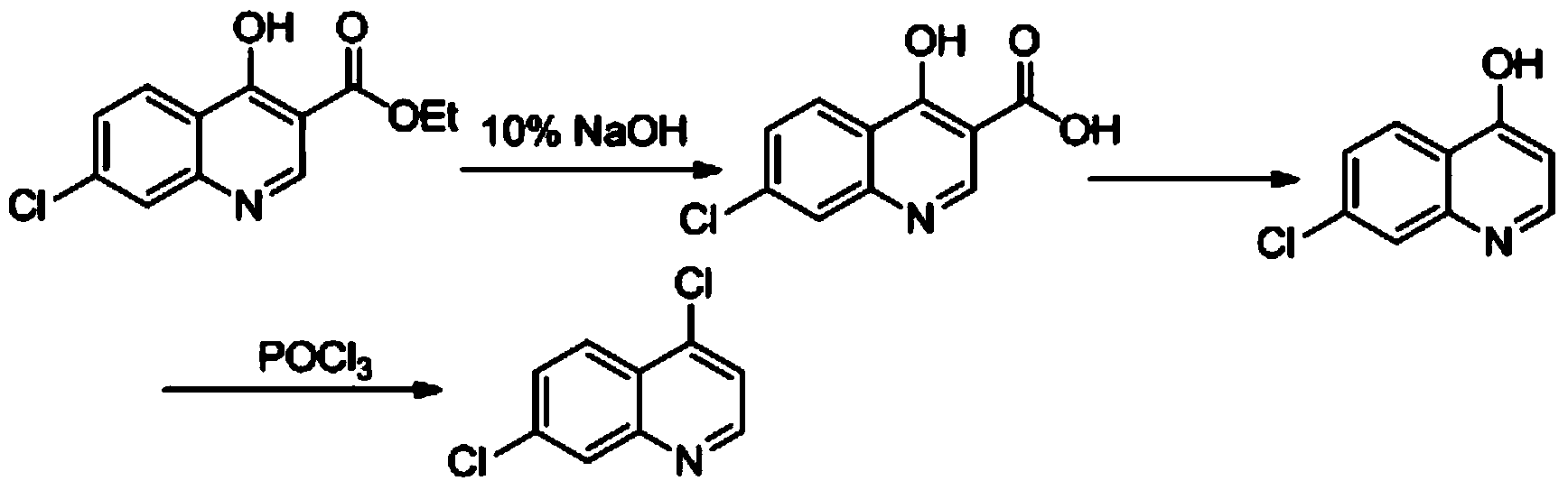
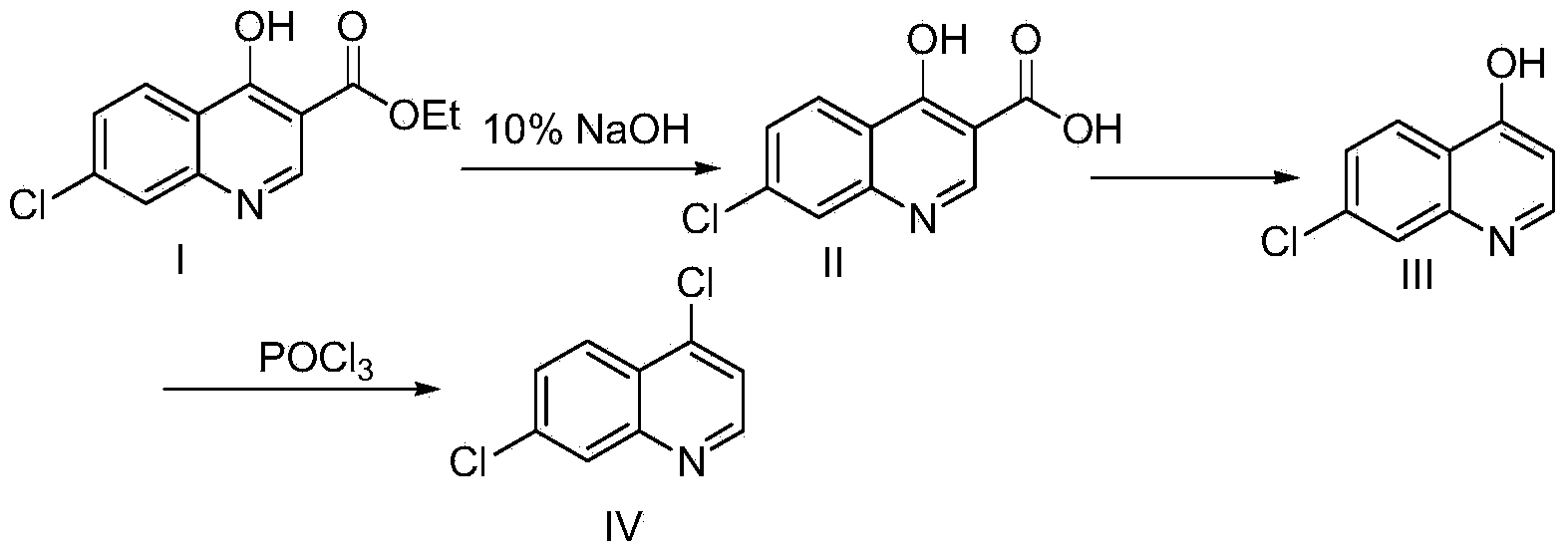
[0017] a, the preparation of compound 4-hydroxyl-7 chloro-quinoline-3-carboxylic acid
[0018] Add 50g of ethyl 4-hydroxy-7-chloro-quinoline-3-carboxylate into a 250mL single-necked bottle, then add 120g of 10% NaOH solution, stir and heat to 90-100°C to dissolve, then add activated carbon for half an hour to decolorize, and filter with suction , the filtrate was adjusted to PH to 3-4 with 10% HCl solution, and a large amount of solids were precipitated, cooled to room temperature, filtered with suction, washed with water, and dried to obtain 40.87 g of the product, with a yield of 92%
[0019] B, the preparation of compound 4-hydroxyl-7-chloroquinoline
[0020] Add 120 mL of paraffin oil into a 250 mL three-necked flask, stir, and add 40 g of 4-hydroxy-7-chloro-quinoline-3 carboxylic acid. Heat to raise the temperature to 230-250 and react for 30 minutes. After the reaction, cool to room temperature and suction filter, wash and dry with toluene to obtain 31.5 g of 7-chloro-4...
Embodiment 2
[0024] a, the preparation of compound 4-hydroxyl-7 chloro-quinoline-3-carboxylic acid
[0025] Add 50g of ethyl 4-hydroxy-7-chloro-quinoline-3-carboxylate into a 250mL single-necked bottle, then add 120g of 10% NaOH solution, stir and heat to 90-100°C to dissolve, then add activated carbon for half an hour to decolorize, and filter with suction , the filtrate was adjusted to PH to 3-4 with 10% HCl solution, and a large amount of solids were precipitated, cooled to room temperature, filtered with suction, washed with water, and dried to obtain 42.2 g of the product, with a yield of 95%
[0026] B, the preparation of compound 4-hydroxyl-7-chloroquinoline
[0027] Add 120 mL of paraffin oil into a 250 mL three-necked flask, stir, and add 40 g of 4-hydroxy-7-chloro-quinoline-3 carboxylic acid. Heat to raise the temperature to 230-250°C, react for 30 minutes, cool to room temperature after the reaction, filter the toluene with suction to wash the filter cake, and dry to obtain 32....
Embodiment 3
[0031] a, the preparation of compound 4-hydroxyl-7 chloro-quinoline-3-carboxylic acid
[0032] Add 50g of ethyl 4-hydroxy-7-chloro-quinoline-3-carboxylate into a 250mL single-necked bottle, then add 120g of 10% NaOH solution, stir and heat to 90-100°C to dissolve, then add activated carbon for half an hour to decolorize, and filter with suction , the filtrate was adjusted to PH with 10% HCl solution until the Congo red test paper turned blue, and a large amount of solid was precipitated, cooled to room temperature, filtered with suction, washed with water, and dried to obtain 41.32 g of the product, with a yield of 93%
[0033] B, the preparation of compound 4-hydroxyl-7-chloroquinoline
[0034] Add 120 mL of paraffin oil into a 250 mL three-necked flask, stir, and add 40 g of 4-hydroxy-7-chloro-quinoline-3-carboxylic acid. Heat to raise the temperature to 230-250 and react for 30 minutes. After the reaction, cool to room temperature and suction filter, wash and dry with tolu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com