Combined drug and applications of combined drug as immuno-regulation agent
An immunomodulatory agent and drug technology, applied in the directions of drug combinations, antipyretics, anti-inflammatory agents, etc., can solve the problem of not finding hydroxychloroquine sulfate and artemisinin, and achieve the effect of reducing the amount of use and reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
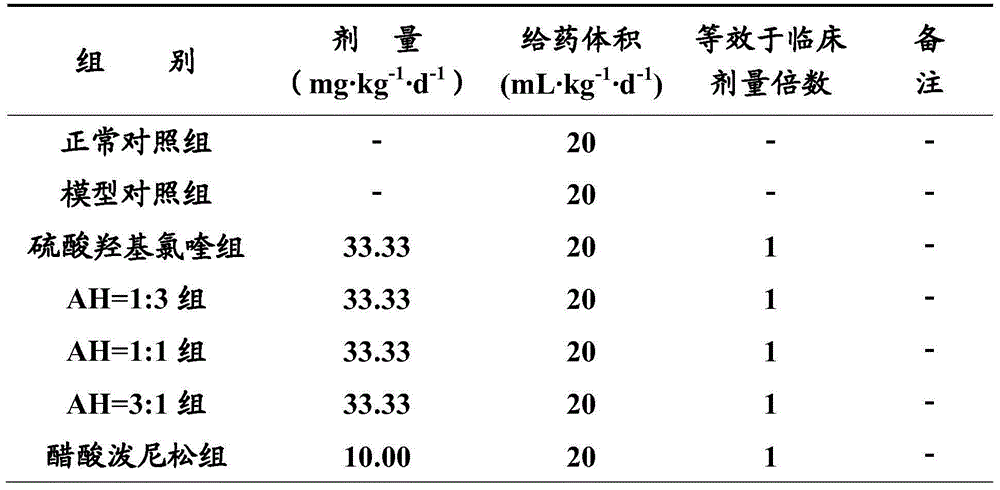
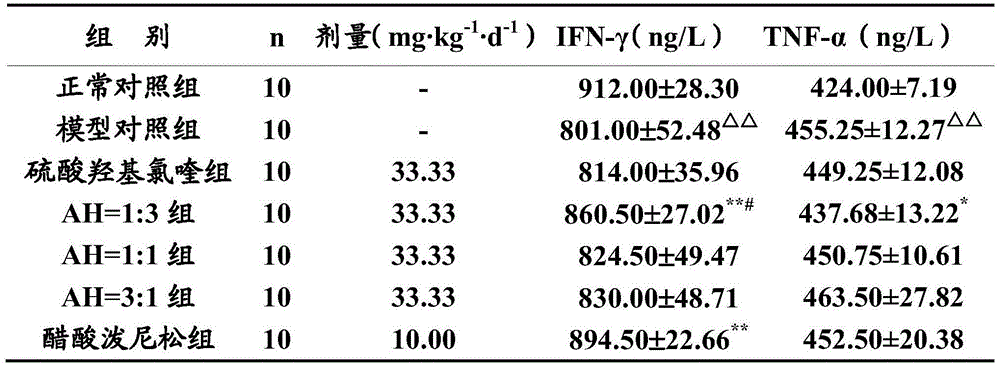
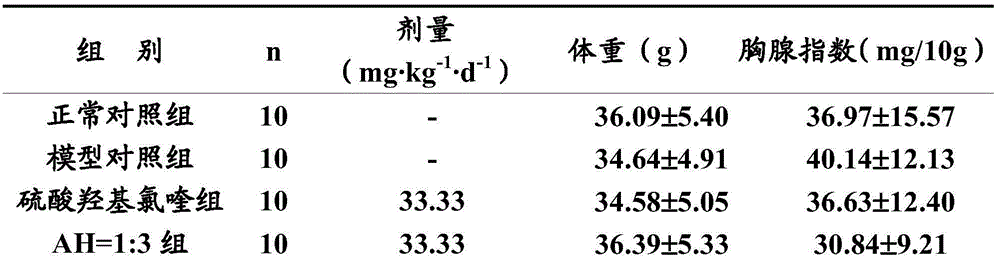
[0020] Experimental Example 1 Pharmacodynamics Experiment of Artemisinin and Hydroxychloroquine Sulfate in Combination Treatment of Immune Diseases
[0021] Hydroxychloroquine sulfate (Hydroxychloroquine Sulphate, HCQ, hereinafter referred to as H) is an important drug for clinical treatment of immune diseases, but it has obvious toxic and side effects, which limits its clinical application. In this experiment, artemisinin (Artemisinin, ART, hereinafter referred to as A) and hydroxychloroquine sulfate (hereinafter referred to as AH) were used to reduce the dosage of hydroxychloroquine sulfate, and the peripheral blood cytokines, cellular immune function, humoral immune function, non-specific Several aspects of immune function were used to evaluate the efficacy of AH after compatibility, and to compare it with that of hydroxychloroquine sulfate alone. The research results of this experiment are of great significance to the development of clinical application of AH in the treatm...
experiment example 2
[0152] Experimental Example 2 Artemisinin Acute Toxicity Test
[0153] To observe and study the toxic reaction of Kunming (KM) mice given artemisinin three times within 24 hours, within a certain period of time, and to understand the dose of acute toxicity.
[0154] 1. Materials
[0155] 1.1 Test product
[0156] 1.1.1 Artemisinin (Artemisinin, ART, hereinafter referred to as "A"): Sichuan Tongrentai Pharmaceutical Co., Ltd., batch number: 130602, purity 98.8%;
[0157] 1.1.2 Hydroxychloroquine Sulphate (Hydroxychloroquine Sulphate, HCQ, hereinafter referred to as "H"): Chongqing Kangle Pharmaceutical Co., Ltd., batch number: SQK-130403, purity 98.7%, base content 77.40%.
[0158] 1.2 Drug preparation
[0159] Use BS210S electronic balance (1 / 10,000) to weigh, add 2 to 3 drops of Tween 80 in a mortar, fully grind to make a suspension, and then dilute with distilled water to the concentration required for the experimental design. Pre-drug preparation.
[0160] 1.3 Animals ...
experiment example 3
[0172] Experimental example 3 hydroxychloroquine sulfate acute toxicity test
[0173] To observe and study the toxic reaction of hydroxychloroquine sulfate given to KM mice once by intragastric administration within a certain period of time, and to understand the dose of acute toxicity.
[0174] 1. Materials
[0175] Same as experimental example 2.
[0176] 2. Experimental method
[0177] D by pretest m (Maximum dose for 100% death) and Dn (minimum dose for 0% death) result, D m Take 4000mg / kg, D n Take 1500mg / kg, the group interval is 1:0.78, KM mice are randomly divided into 5 administration groups [4000mg / kg, 3120mg / kg, 2434mg / kg, 1898mg / kg, 1481mg / kg] and 1 vehicle control Group (purified water), 10 rats in each group, 5 rats for each ♀♂. Each group was intragastrically administered once at 10 mL / kg body weight; the symptoms, onset time, duration, death time, and near-death reaction of the mice were observed continuously for 6 hours after the administration. After t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ld50 | aaaaa | aaaaa |
| Ld50 | aaaaa | aaaaa |
| Ld50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com