Preparation method of hydroxychloroquine and sulfate thereof
A technology of hydroxychloroquine and hydroxychloroquine sulfate, applied in organic chemistry methods, organic chemistry, etc., can solve the problems of unstable impurity control and low purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
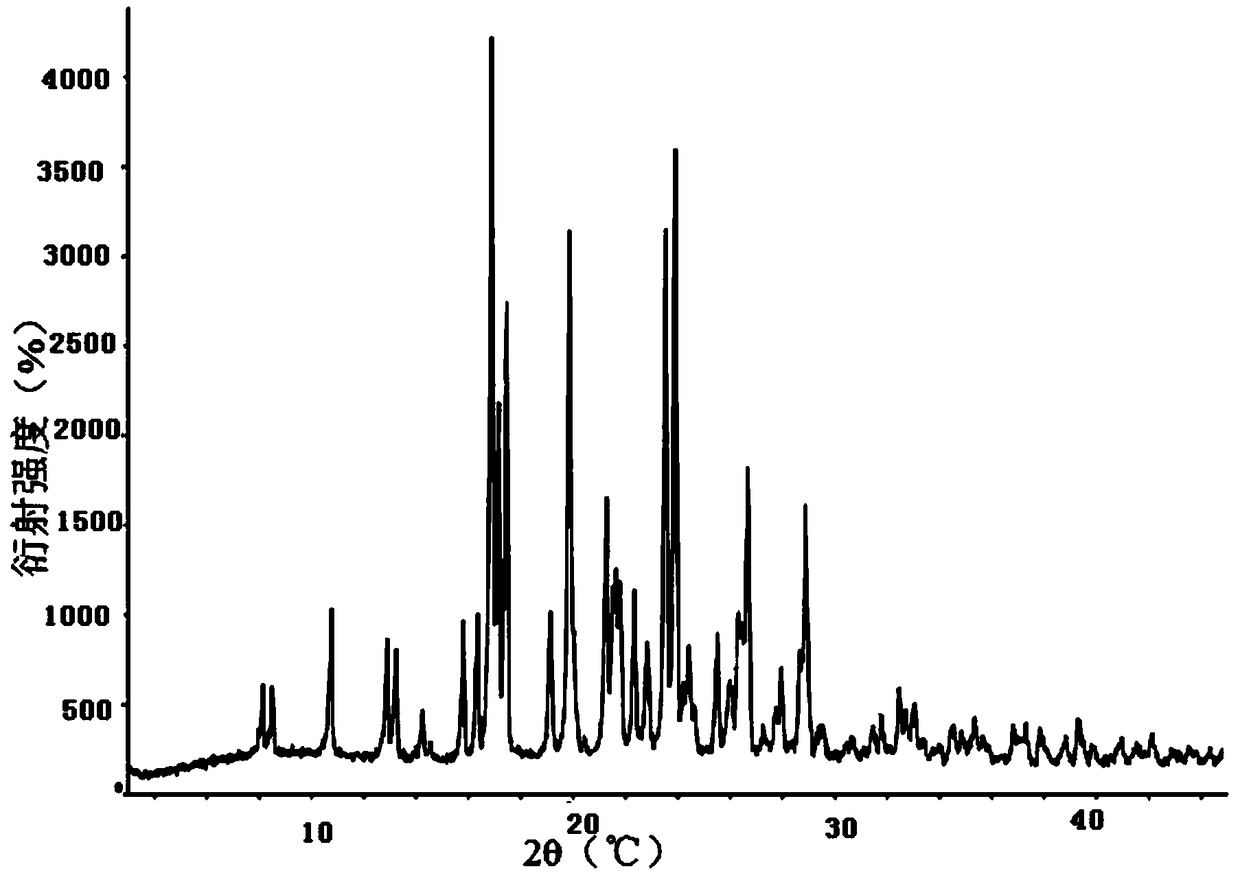
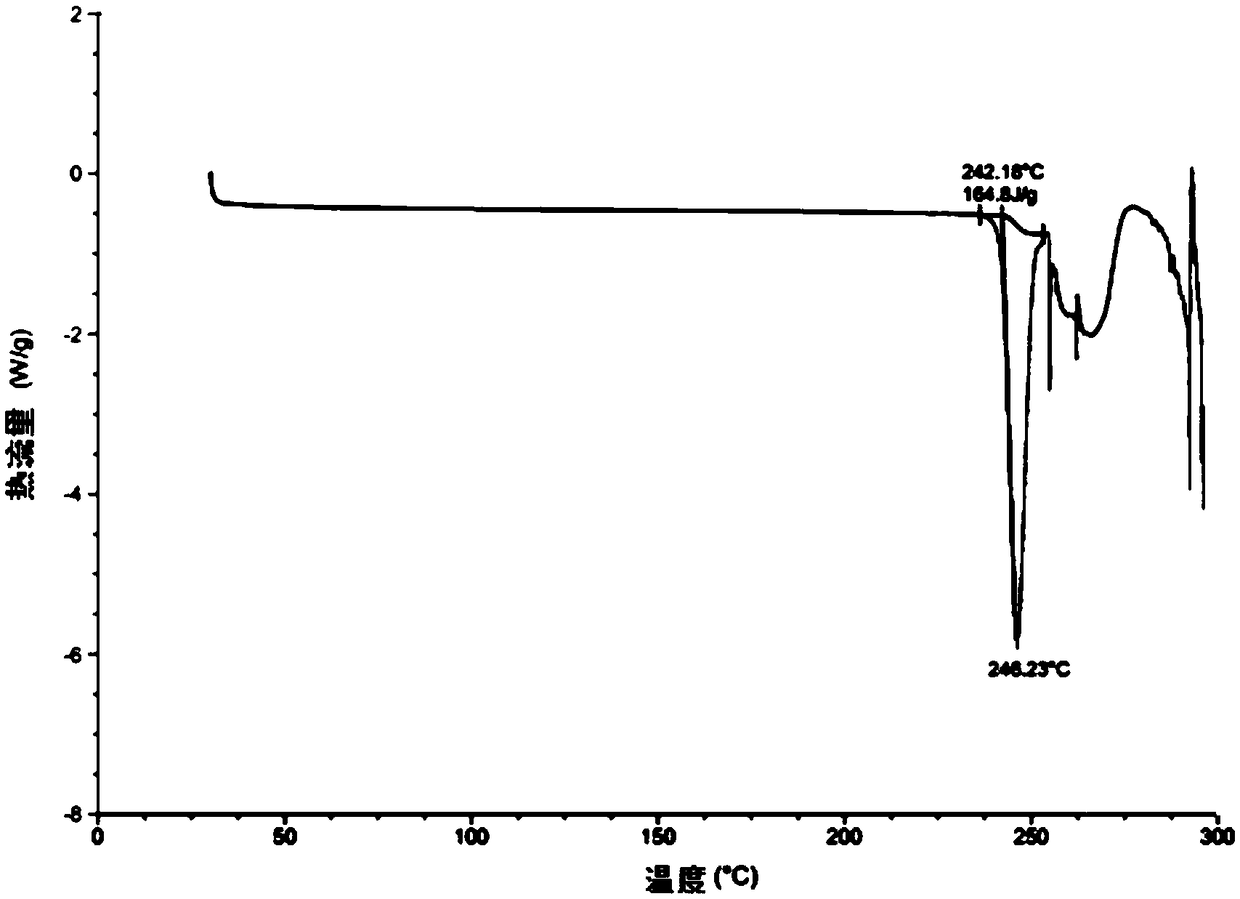
Image
Examples
Embodiment 1~8
[0090] The preparation of step (1), hydroxychloroquine crude product
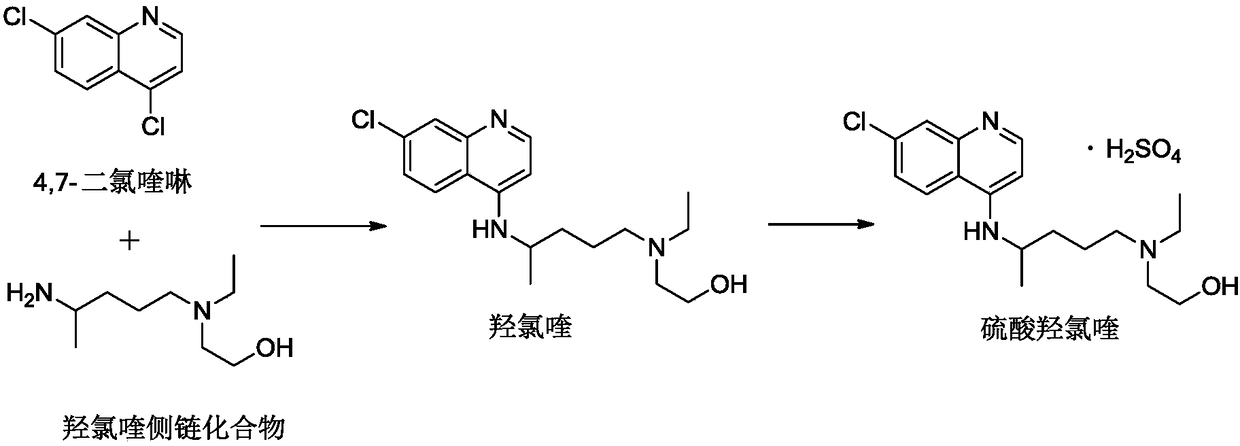
[0091] Add 4,7-dichloroquinoline (100g) and side chains into the reaction flask, under nitrogen protection, stir, rise to a certain temperature, react, until HPLC detects that the content of 4,7-dichloroquinoline is below 6%, stop For the reaction, quench the reaction with a quenching liquid, extract with an organic solvent, wash the extract with purified water until the pH is 7-8, and distill under reduced pressure to obtain crude hydroxychloroquine.
[0092] Table 1. Preparation of hydroxychloroquine crude product
[0093]
[0094] The preparation of step (2), hydroxychloroquine fine product
[0095] Add the mixed solvent to the crude product obtained in step (1), heat to 70±5°C to dissolve, then lower the temperature, cool to 10°C, add seed crystals, crystallize, suction filter, and dry the filter cake to obtain fine hydroxychloroquine.
[0096] Table 2. Preparation of Hydroxychloroquine Essence
[0...
Embodiment 1 and comparative Embodiment 3
[0121] Embodiment 1 and comparative example 3,4 compare
[0122] Table 3. Comparison of products and impurities in crude hydroxychloroquine
[0123]
[0124] Table 4. Comparison of products and impurities in hydroxychloroquine boutique
[0125]
[0126]
[0127] Note: The relative retention time is compared with the HPLC retention time of hydroxychloroquine; that is, a relative retention time of "1" means hydroxychloroquine, and other relative retention times are impurities.
[0128] After refining, in Example 1, the content of impurity 1 is controlled at 0.1% unqualified in the subsequent storage process.
Embodiment 9~17 and comparative Embodiment 9~11
[0130] Preparation of hydroxychloroquine sulfate-salt crystallization
[0131] Hydroxychloroquine fine product (50g; obtained by Example 1) was dissolved in an alcoholic solvent, and sulfuric acid aqueous solution was added dropwise at 20 to 35° C. until turbidity, and the dropwise addition was stopped, and the temperature was raised to 35 to 55° C., and the heat preservation reaction was carried out for more than 5 hours. After the reaction is completed, the temperature is lowered to 0-20° C., kept for 1 hour, filtered with suction, and the filter cake is dried to obtain the finished product.
[0132] Table 5. Preparation of hydroxychloroquine sulfate
[0133]
[0134]
[0135] Wherein, the HPLC data analysis of embodiment 11 and comparative example 9 is as follows:
[0136] Table 6. Comparison of products and impurities in hydroxychloroquine sulfate
[0137]
[0138] Note: The relative retention time is compared with the HPLC retention time of hydroxychloroquine s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com