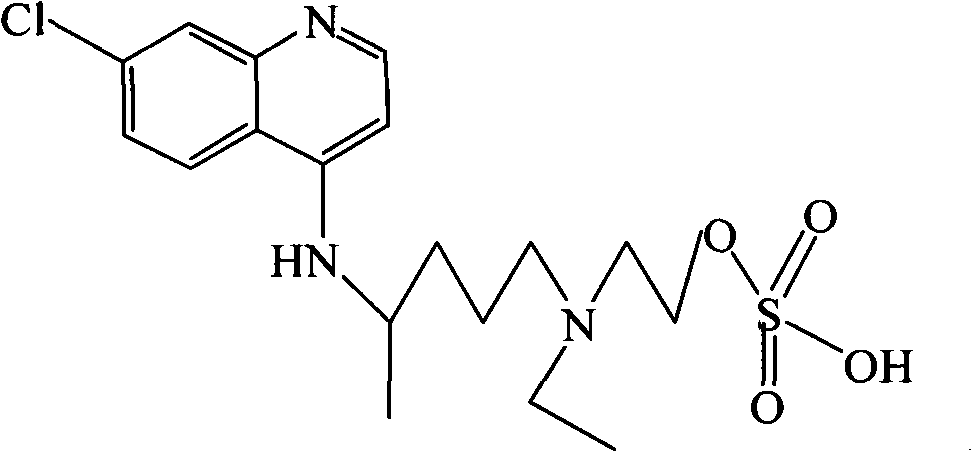
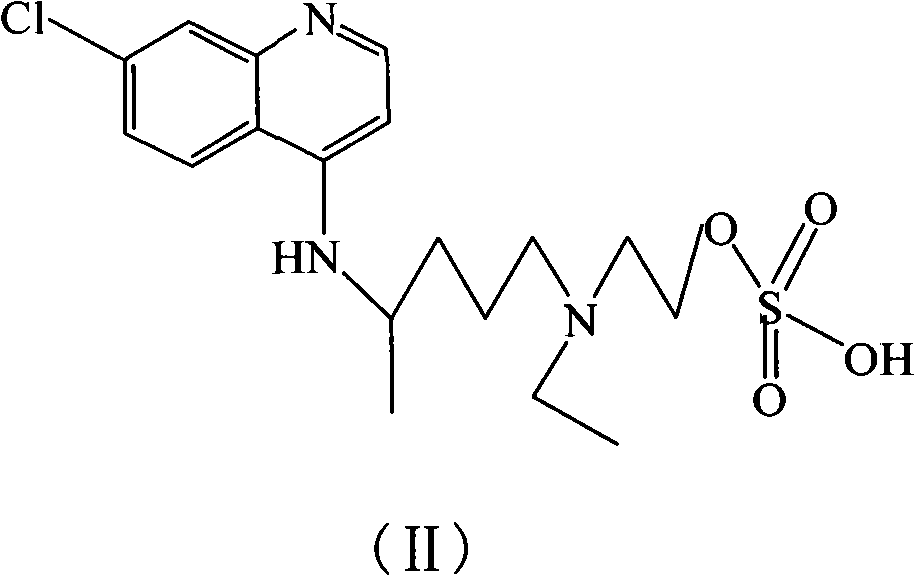
Hydroxychloroquine derivative and its synthetic method
A technology of hydroxychloroquine and its synthetic method, which is applied in the field of 2-[[4-[amino]pentyl]ethylamino]-ethylsulfate and hydroxychloroquine derivatives II, and can solve the problems of less public information, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
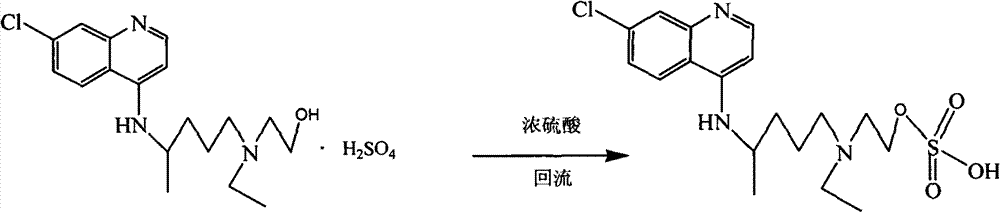
[0026] The synthetic method 1 of embodiment 1 compound II
[0027] In a three-necked reaction flask, add 20g of hydroxychloroquine sulfate, 40mL of concentrated sulfuric acid, and 200mL of dichloromethane, stir, heat to reflux, and react for 10 hours. After the reaction is complete, the reaction solution is poured into 500mL of water, and adjusted to neutrality with Extracted with dichloromethane, the organic phase was spin-dried, 100mL ethyl acetate was added to the residue, stirred at room temperature for 12h, a large amount of solid was precipitated, filtered by suction, and dried to obtain 17.4g of off-white solid, which was compound II, with a yield of 70.2%, HPLC The peak area normalized method content is 99.5%.
Embodiment 2
[0028] The influence of embodiment 2 different solvents on the preparation yield, quality of compound II
[0029] According to the synthetic method 1 of compound II, the selected solvents are respectively: dichloromethane, chloroform, toluene, DMF, completed 4 experiments, obtained 4 samples, and its yield and quality data are shown in the following table:
[0030] sample
[0031] Conclusion: Dichloromethane is used as the reaction solvent, and the yield and quality are relatively the best.
Embodiment 3
[0032] The influence of embodiment 3 different solid precipitation reagents on the preparation yield, quality of compound II
[0033] According to the synthetic method 1 of compound II, the selected solid precipitation solvents are respectively: ethyl acetate, acetone, petroleum ether, n-hexane, 4 experiments are completed, and 4 samples are obtained. The yield and quality data are shown in the following table:
[0034] sample
[0035] 2
[0036] Conclusion: Ethyl acetate is used as a reagent for solid precipitation, and the yield and quality are relatively the best.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com