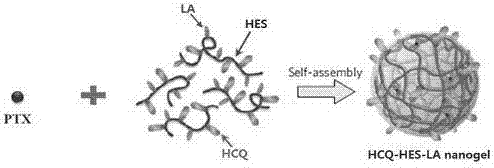
Chloroquine-polymerized nanogel delivery system and preparation method thereof
A nanogel and delivery system technology, applied in the field of chemotherapy drug delivery system and its preparation, can solve the problems of inability to deliver multiple substances at the same time, inability to achieve multifunctional modification, large particle size, etc., and achieve controllable loading capacity. , Inhibition of cancer cell proliferation, the effect of controllable amount of modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
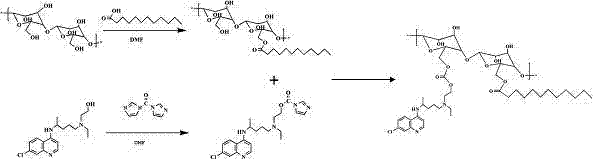
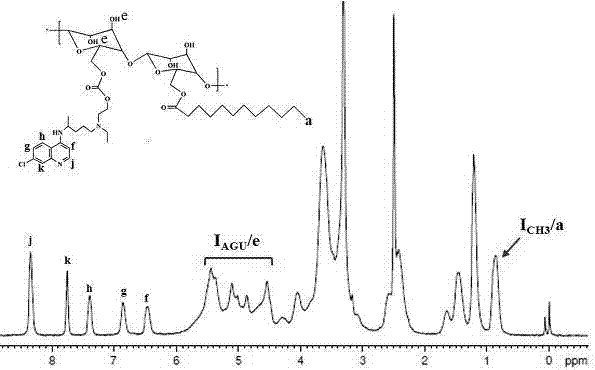
[0051] Embodiment 1 A kind of preparation method of hydroxychloroquine-lauric acid-hydroxyethyl starch
[0052] (1) Synthesis of hydroxychloroquine: Dissolve 1 g of hydroxychloroquine sulfate in 2 ml of pure water, add 2 ml of 30% ammonia water dropwise under constant stirring until a white insoluble product is produced, add dichloromethane to extract the aqueous phase 4-6 Once, the organic phase was collected and anhydrous sodium sulfate was added overnight, and the solvent was distilled off under reduced pressure to obtain the final desalted product hydroxychloroquine.
[0053] (2) Synthesis of lauric acid-hydroxyethyl starch: Dissolve 100 mg of hydroxyethyl starch in 2 ml of dimethyl sulfoxide, add 124 mg of dicyclohexylcarbodiimide and 127 mg of 4-dimethylaminopyridine and 30 mg of lauric acid, reacted for 12-18 hours under the protection of an inert gas, and removed the insoluble matter by suction filtration, then added the reaction solution to 10 times the amount of anhy...
Embodiment 2
[0056] Embodiment 2 A kind of preparation method of hydroxychloroquine-lauric acid-hyaluronic acid
[0057] (1) Synthesis of hydroxychloroquine: Dissolve 1 g of hydroxychloroquine sulfate in 2 ml of pure water, add 2 ml of 30% ammonia water dropwise under constant stirring until a white insoluble product is produced, add dichloromethane to extract the aqueous phase 4-6 Once, the organic phase was collected and anhydrous sodium sulfate was added overnight, and the solvent was distilled off under reduced pressure to obtain the final desalted product hydroxychloroquine.
[0058] (2) Synthesis of lauric acid-hyaluronic acid: Dissolve 100 mg of hyaluronic acid in 2 ml of dimethyl sulfoxide, add 103 mg of dicyclohexylcarbodiimide, 103 mg of 4-dimethylaminopyridine and 25.7 mg lauric acid, react for 12-18 hours under the protection of an inert gas, remove the insoluble matter by suction filtration, add the reaction solution to 10 times the amount of anhydrous ether, filter with sucti...
Embodiment 3
[0060] Embodiment 3 A kind of preparation method of hydroxychloroquine-lauric acid-dextran
[0061] (1) Synthesis of hydroxychloroquine: Dissolve 1 g of hydroxychloroquine sulfate in 2 ml of pure water, add 2 ml of 30% ammonia water dropwise under constant stirring until a white insoluble product is produced, add dichloromethane to extract the aqueous phase 4-6 Once, the organic phase was collected and anhydrous sodium sulfate was added overnight, and the solvent was distilled off under reduced pressure to obtain the final desalted product hydroxychloroquine.
[0062](2) Synthesis of lauric acid-dextran: Dissolve 100 mg dextran in 2 ml dimethyl sulfoxide, add 240 mg dicyclohexylcarbodiimide, 240 mg 4-dimethylaminopyridine and 60 mg Lauric acid, react for 12-18 hours under the protection of an inert gas, remove the insoluble matter by suction filtration, add the reaction solution to 10 times the amount of anhydrous ether, filter with suction, dissolve the solid with ultrapure w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com