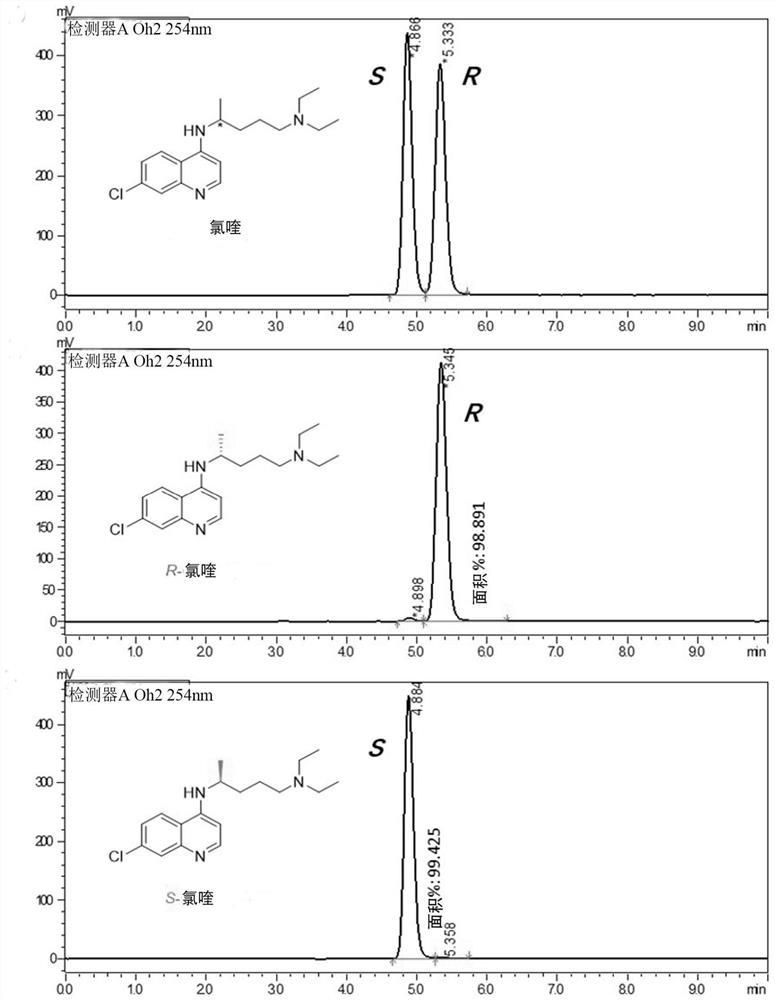
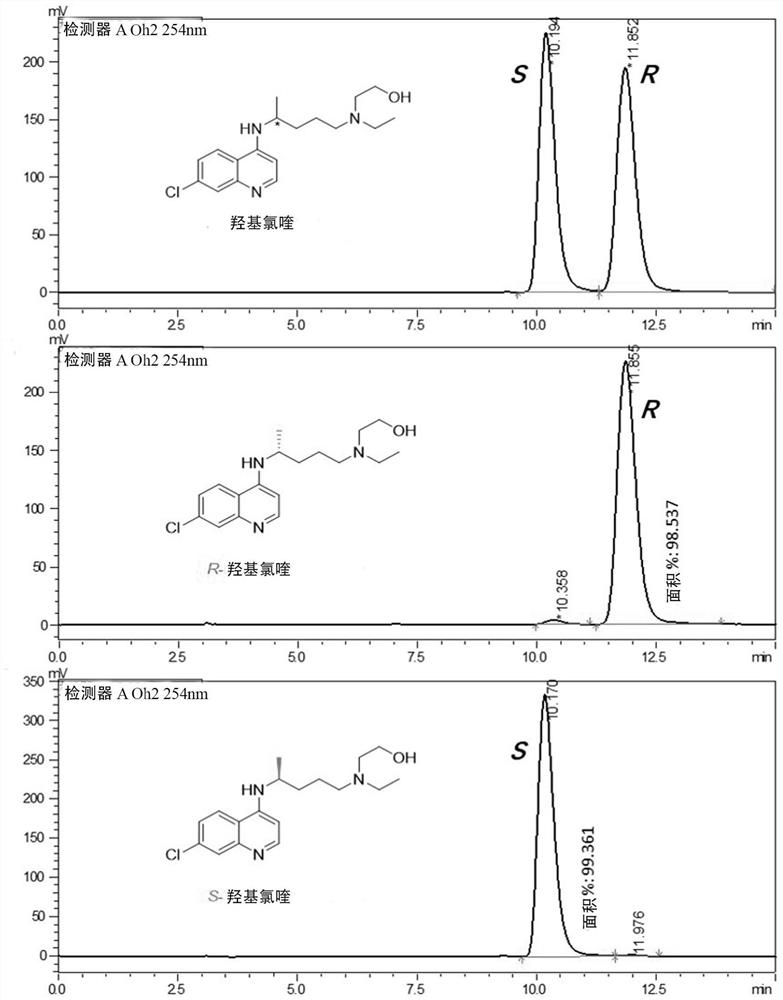
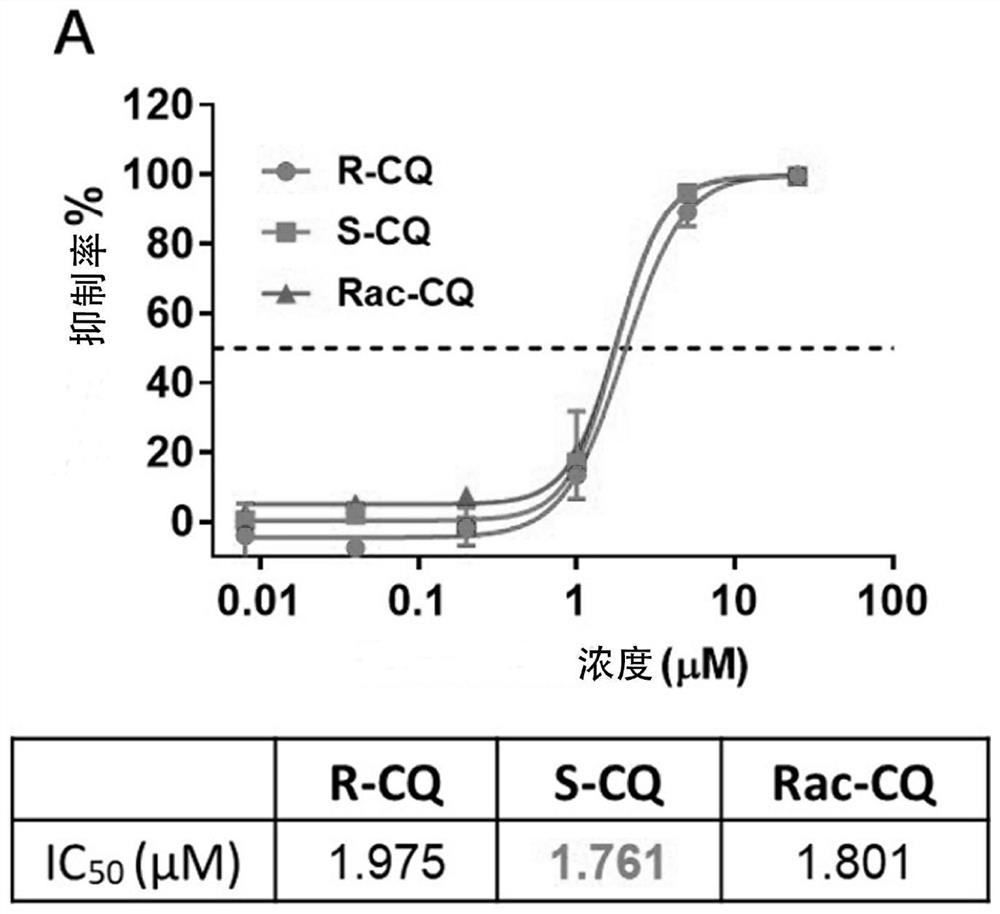
Application of chiral chloroquine, hydroxychloroquine or salt of the chiral chloroquine and hydroxychloroquine as anti-coronavirus drug target 3CL hydrolase inhibitor for reducing cardiotoxicity
A coronavirus and hydrolytic enzyme technology, applied in antiviral agents, drug combinations, medical preparations containing active ingredients, etc., can solve problems such as prolonged QT interval, tachycardia, and sudden death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Preparation of Chiral Chloroquine by Chiral High Performance Liquid Chromatography
[0066] The racemic chloroquine phosphate 1 purchased from the market is converted into free racemic chloroquine 2 under alkaline conditions.
[0067]
[0068] At 0°C, 13.0 g of chloroquine phosphate was dissolved in 75 mL of water, then 50 mL of 12% NaOH aqueous solution was added, after stirring for half an hour, 25 mL of ethyl acetate was added, and stirring was continued for half an hour. The reaction solution was naturally raised to room temperature, extracted three times with 100 mL of ethyl acetate, combined the organic phases, washed with 150 mL of saturated brine and water successively, dried by adding anhydrous sodium sulfate, and filtered to remove sodium sulfate. The organic solvent was removed with a rotary evaporator to obtain 7.6 g of free chloroquine in the form of light yellow viscous liquid, with a yield of 94%.
[0069] 1 H NMR (600MHz, Chloroform-d) δ8.50 (d, J =...
Embodiment 2
[0073] Preparation of Optically Pure Chloroquine Phosphate
[0074] R and S Free State Chloroquine Converted to Optically Pure Chloroquine Phosphate
[0075]
[0076]Dissolve 640 mg of S-chloroquine in 4 mL of ethanol and heat to reflux. 0.25 mL of 85% phosphoric acid was added dropwise to the above solution, and the reaction was refluxed for two hours. At this time, a large amount of white solids were precipitated. After the reaction solution was cooled to room temperature, it was filtered, and the filter cake was washed three times with 1 mL of ethanol to obtain 868 mg of white S-chloroquine phosphate solid, with a yield of 84%, [α] D 27.8 =79.7(c=0.5,H 2 O).
[0077]
[0078] Dissolve 640 mg of R-chloroquine in 4 mL of ethanol and heat to reflux. 0.25 mL of 85% phosphoric acid was added dropwise to the above solution, and the reaction was refluxed for two hours. At this time, a large amount of white solids were precipitated. After the reaction solution was coole...
Embodiment 3
[0080] Preparation of Chiral Chloroquine Phosphate by Chiral Synthesis
[0081] Weigh 72g (0.46mol) of the side chain of chloroquine (chemical name: (±)-2-amino-5-diethylaminopentane), add it to a 250mL round bottom flask, then add 100mL of isopropanol, and stir to dissolve it; Add 36.5 g (0.24 mol) of D-(-)-mandelic acid (R-(-)-mandelic acid) into the reaction flask, stir at room temperature, and a large amount of white solid precipitates; stir for 3 hours to complete the salt formation, filter with suction, and use Wash the solid with isopropanol three times, 50 mL each time; combine the mother liquors and concentrate to 100 mL, add a small amount of the solid obtained by suction filtration as a seed crystal, so that the unprecipitated solid crystallizes; filter with suction, and wash with isopropanol three times, 50 mL each time. Combine the white solids obtained by suction filtration twice, put in a 250mL flask, pour 50mL of isopropanol, stir under reflux, slowly add isopr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com