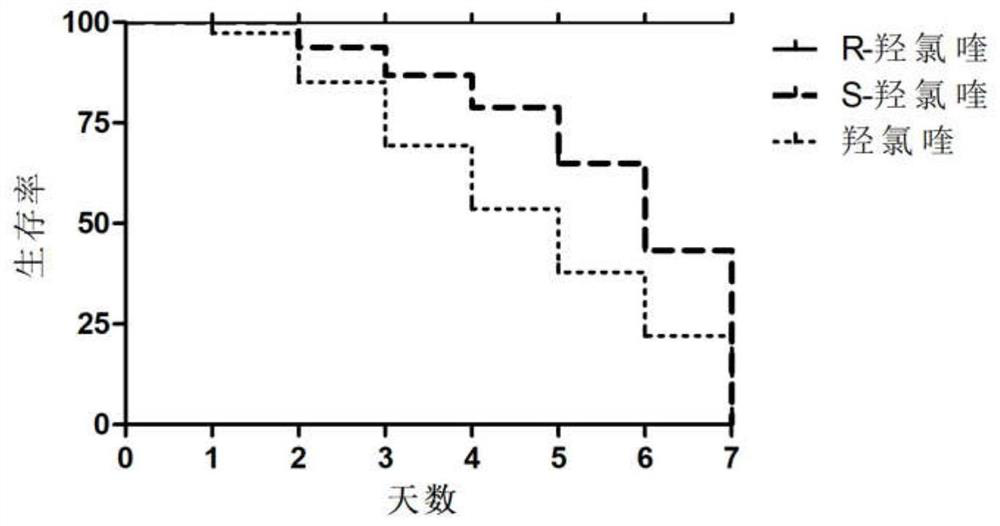
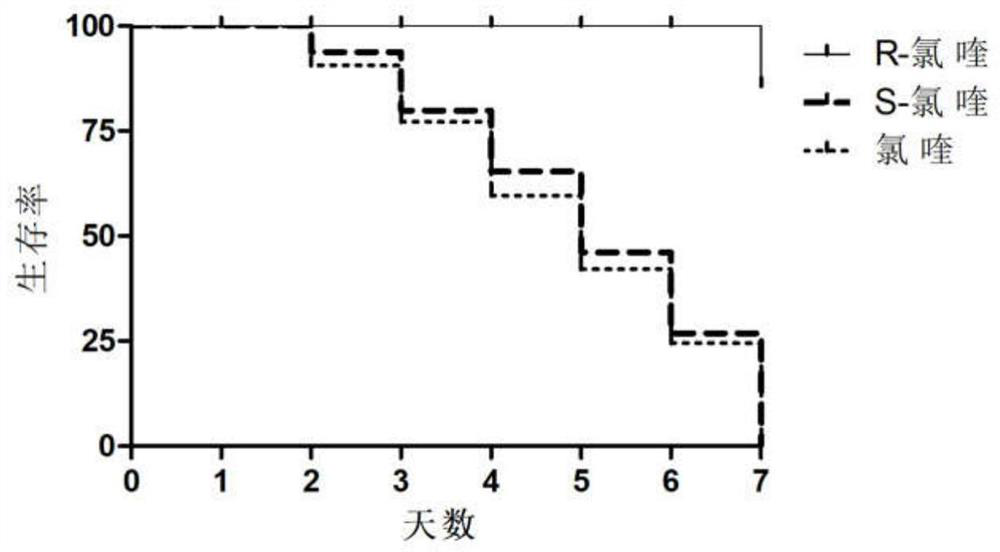
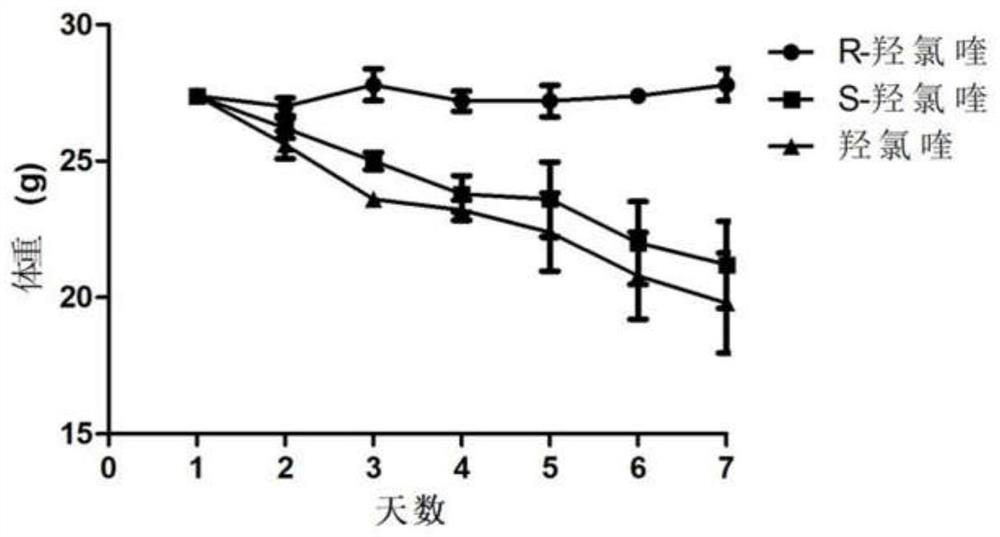
Optically active chloroquine and hydroxychloroquine and analogues thereof, and preparation method, composition and application of optically active chloroquine and hydroxychloroquine
A compound, chiral separation technology, applied in the fields of organic active ingredients, organic chemistry, pharmaceutical formulations, etc., can solve problems such as retinal damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] Embodiment 1: prepare the L-malate of (R)-hydroxychloroquine
[0135] Dissolve racemic hydroxychloroquine (5 g, 14.88 mol) in ethanol / ethyl acetate (v / v=1:2, 30 mL), and dissolve L-malic acid (6 g, 44.64 mol) in ethanol / ethyl acetate Ester (v / v=1:2, 15 mL), at 0°C, slowly dropwise add L-malic acid in ethanol / ethyl acetate solution to the above racemate in ethanol / ethyl acetate solution. When the temperature was raised to 5° C., reacted for 30 minutes, until the reaction was complete, most of the salts of the L-malic acid of (S)-hydroxychloroquine were dissolved in the ethanol / ethyl acetate mixed solution, while the salts of the L-malic acid of (R)-hydroxychloroquine The acid salt was precipitated, and the L-malate crude product (5.2 g) of (R)-hydroxychloroquine was obtained by suction filtration.
Embodiment 2
[0136] Embodiment 2: the L-malate recrystallization purification of (R)-hydroxychloroquine
[0137] The L-malate crude product (5.2 g) of (R)-hydroxychloroquine was suspended in 10 mL of ethanol / ethyl acetate (v / v=1:2), heated to reflux until completely dissolved, slowly cooled to room temperature, and Keep stirring for 1-2 hours, and continue to cool down to 5-10° C., stir for 30 minutes, and filter with suction to obtain (R)-hydroxychloroquine L-malate (4.3 g) with an optical purity of 87.8% ee. Then, according to the above recrystallization method, the L-malate of (R)-hydroxychloroquine obtained above was recrystallized once to obtain the L-malate of (R)-hydroxychloroquine with an optical purity of 98.8% ee. (3.8g).
Embodiment 3
[0138] Embodiment 3: the preparation of (R)-hydroxychloroquine
[0139]
[0140] Sodium methoxide (1g, 20.24mol) was added in batches to 10ml of anhydrous methanol. After stirring for about 10min at 0°C, the system became clear, and (R)-hydroxychloroquine L-malate (3.8g , 5.06mmol), stirred at room temperature for 30min, concentrated under reduced pressure to remove methanol. Add 20ml of ethyl acetate, stir at room temperature for 1 hour, desalt by suction filtration, concentrate the ethyl acetate mother liquor under reduced pressure to obtain (R)-hydroxychloroquine (1.6g), purity>99%, chiral purity>99.92%, yield 64%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com