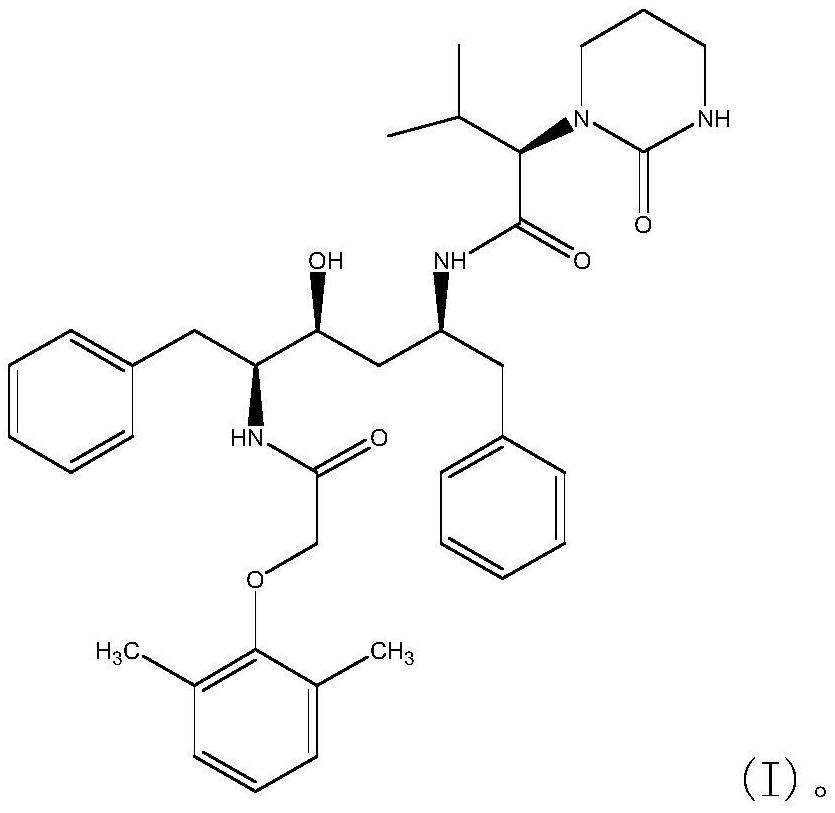
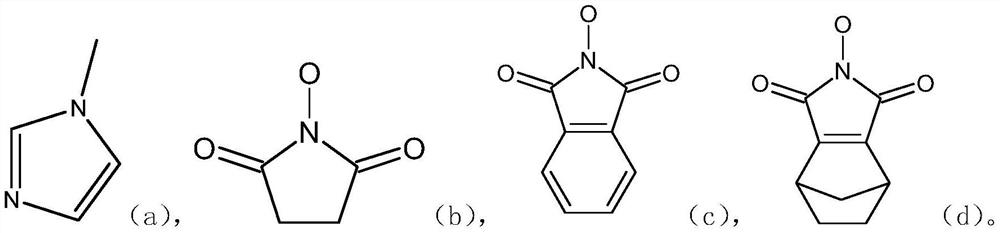
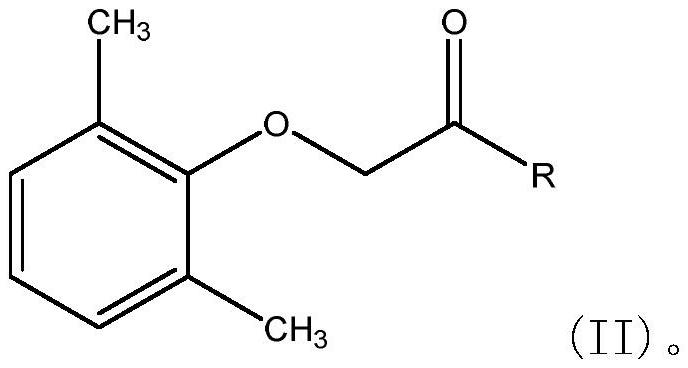
One-pot preparation of lopinavir
A carboxyl activator and condensation reaction technology, applied in the field of medicine, can solve the problems of cumbersome operation and post-processing, increase the reaction steps of activated ester, not suitable for industrial production, etc., and achieve the effect of saving process and fewer synthesis steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053]
Embodiment 1-1
[0055] Disperse 21.6g (0.12mol) of 2,6-dimethylphenoxyacetic acid in 150ml of dichloromethane to form a suspension, add 17.8g of CDI (0.11mol) solid to the reaction solution in batches at room temperature at 25°C, add After stirring at 25°C for 2h, 59.5g (0.1mol) THP was added to the reaction solution, and stirring was continued at 25°C for 8h until the reaction was complete. Quench the reaction with 2g (0.02mol) N,N-dimethyl-1,3-propanediamine, continue to stir at room temperature for 2h, and use 5% citric acid solution 100ml, 5% sodium bicarbonate solution 100ml, 50ml Washed with water 3 times, the organic phase was concentrated under vacuum, the concentration temperature did not exceed 50°C, to obtain 60.3g (96% yield) of lopinavir.
Embodiment 1-2
[0057] Dissolve 21.6g (0.12mol) of 2,6-dimethylphenoxyacetic acid in 60ml of DMF to form a solution, add 17.8g (0.11mol) of CDI in batches at 25°C, and stir at room temperature for 1h. Add 59.5 g (0.1 mol) THP to the reaction solution at 25°C, and continue to stir at room temperature for 8 h until the reaction is complete. The reaction solution was quenched with 2g (0.02mol) N,N-dimethyl-1,3-propanediamine, continued to stir at room temperature for 2h, added 200ml of 5% citric acid to the reaction solution, cooled to 5°C, and stirred for 2h Crystallize, filter, wash the filter cake with 5% citric acid solution, 5% sodium bicarbonate solution and water, and dry the filter cake under vacuum at 50° C. to obtain 58.4 g (93% yield) of lopinavir.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com