Compositions comprising lopinavir and treatment of conditions
A composition and disease technology, applied in the direction of drug combination, medical preparations containing active ingredients, skin diseases, etc., can solve problems such as no effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0363] Example 1: Activity of lopinavir alone and 12:1 (w / w) lopinavir:ritonavir on HSV2 replication.
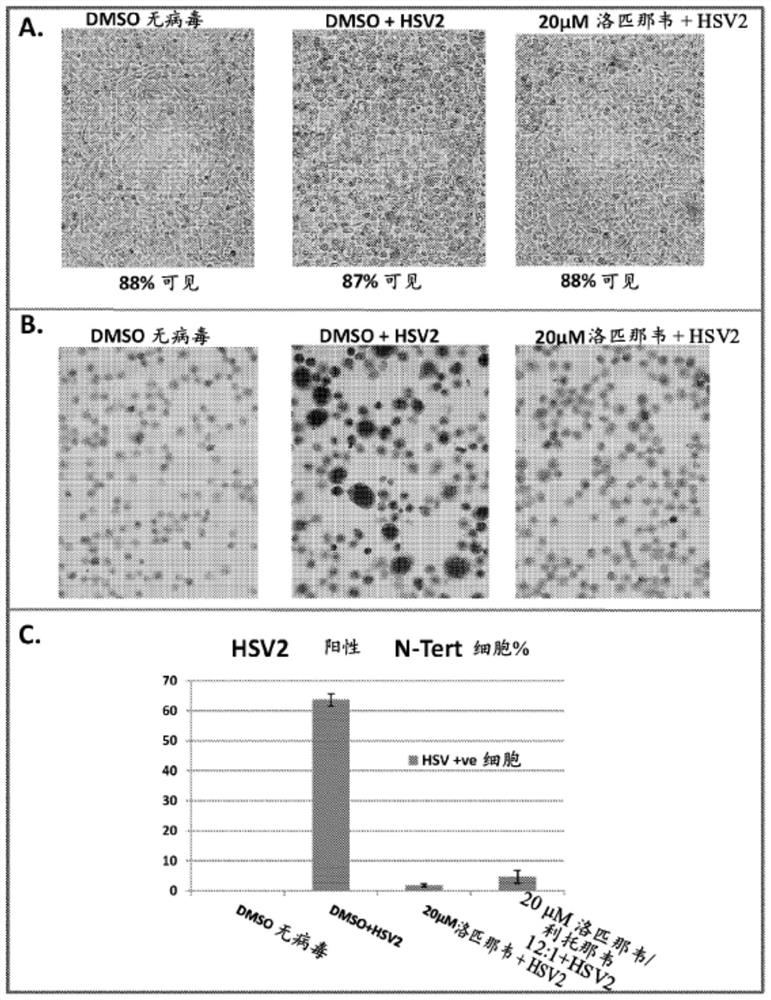
[0364] figure 1 A shows phase contrast images of the effect of HSV2 infection on the morphology of N-Tert keratinocytes exposed to DMSO control or 20 μM lopinavir for 48 hours. The cytopathic effect of the virus was clearly visible in HSV2-infected cells treated with DMSO when compared to uninfected controls. However, HSV2-infected cells treated with 20 μM lopinavir showed little cytopathic effect.
[0365] figure 1 B shows a graph of anti-HSV antibody immunostaining of these cells, demonstrating that the majority of HSV2-infected cells treated with DMSO stained strongly positive for the virus, whereas there was no signal in control virus-negative cells. Very few HSV2 positive cells were seen in infected cells exposed to 20 μM lopinavir for 48 hours.
[0366] figure 1 C shows quantification of the percentage of HSV2 positive cells detected by immunostaining. Analysis o...
example 2
[0367] Example 2: Activity of 12:1w / w Lop / Rit and nelfinavir on HSV2 replication.
[0368] While prior art (Gantt et al. (supra)) suggests that these compounds (lopinavir (alone or with ritonavir)) are ineffective, the HIV protease inhibitor nelfinavir may have When active, the inventors surprisingly found that lopinavir (alone or together with ritonavir) cleared the virus. Therefore, the inventors compared the activity of nelfinavir and the activity of the composition of 12:1 (w / w) lopinavir: ritonavir, and they were studying the use of these APIs in cancer therapy. This compound has been used for efficacy (unpublished work discussed above).
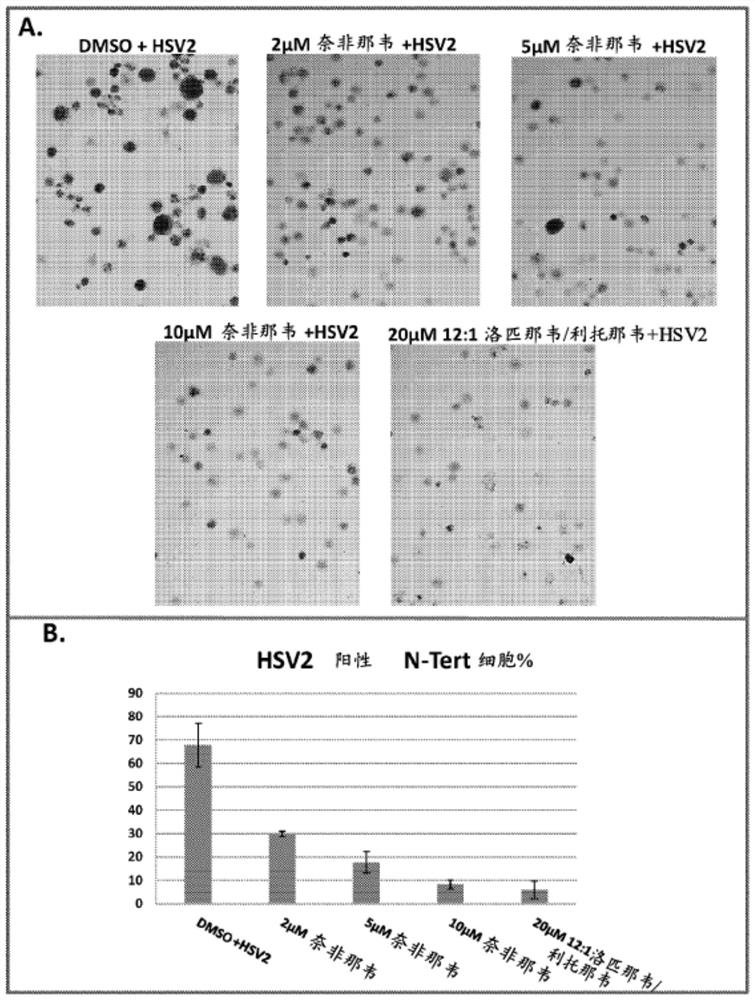
[0369] figure 2 A shows representative images of anti-HSV immunostained HSV2-infected N-Tert keratinocytes treated with the indicated concentrations of API as well as mixtures of different APIs. Three separate images from each treatment were analyzed.
[0370] figure 2 B shows that 2 μM and 5 μM nelfinavir are less effective on H...
example 3
[0371] Example 3: Toxicity of lopinavir alone, Lop / Rit and nelfinavir in virus negative N-Tert cells.
[0372] The inventors noticed that nelfinavir seemed to cause some toxicity to N-Tert host cells and decided to analyze the toxicity.
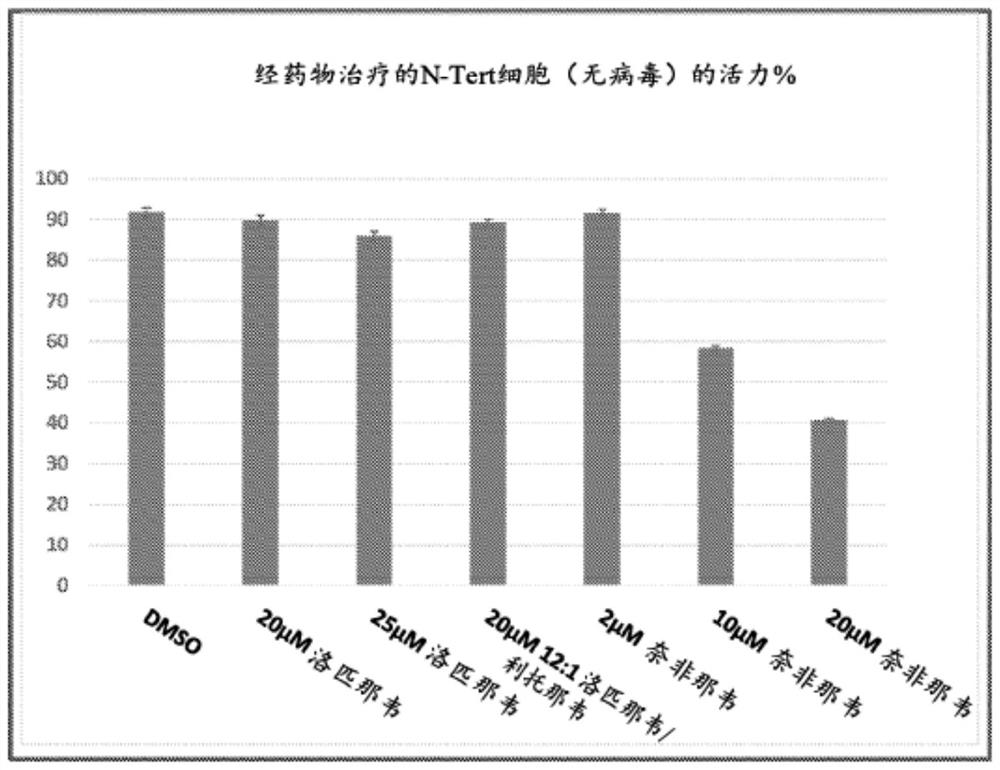
[0373] image 3 Via-1 cassette viability assays are shown for uninfected N-Tert keratinocytes treated for 48 hours with the indicated API concentrations and mixtures of the indicated APIs. As can be seen, up to 25 μM lopinavir alone or 20 μM 12:1 (w / w) lopinavir:ritonavir had little toxic effect on these cells, while 10 μM nelfinavir showed Significant toxicity was observed (reduced cell viability to <60%) and 20 μM nelfinavir showed stronger toxicity (reduced cell viability to about 40%).
[0374] in conclusion
[0375] These results indicated that 20 μM lopinavir or 12:1 w / w Lop / Rit was more effective on HSV2 replication than 2 μM or 5 μM nelfinavir and was comparable to 10 μM nelfinavir. However, 10 μM nelfinavir exhibited stronger non...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com