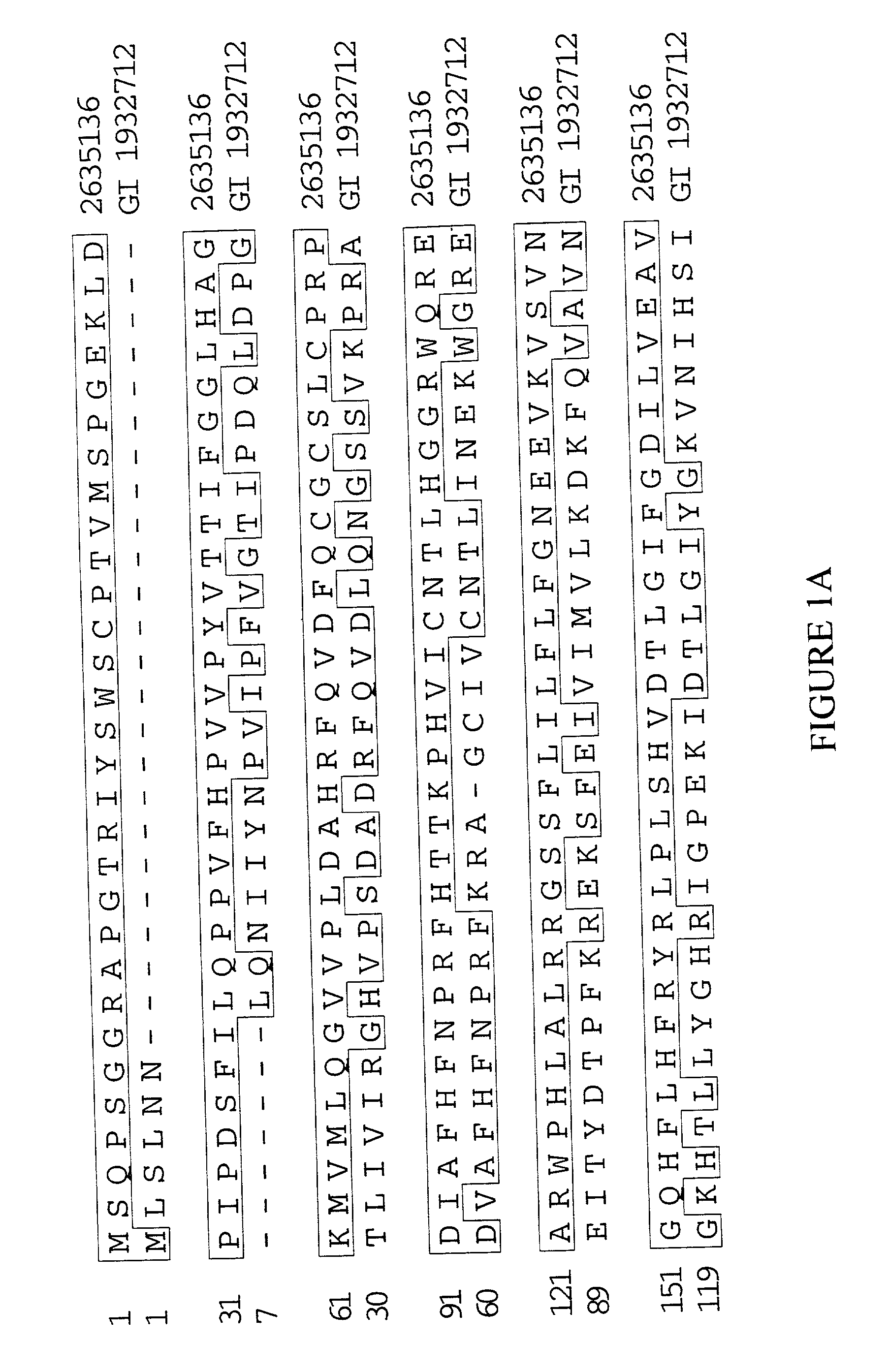
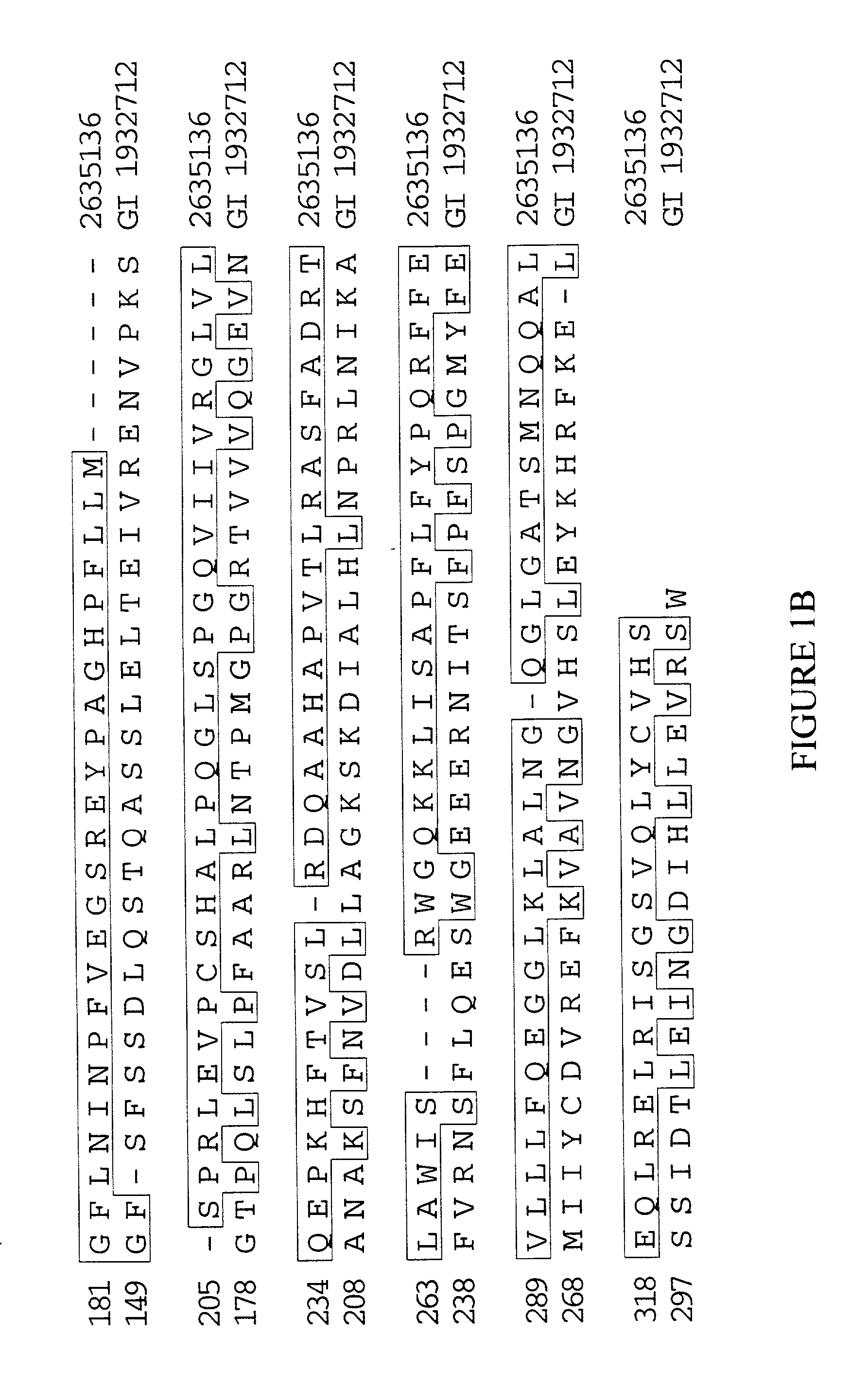
Extracellular adhesive proteins
a technology of adhesive proteins and proteins, applied in the field of extracellular adhesive proteins, can solve the problems of early embryonic lethality in mice, and achieve the effects of increasing the solubility of compounds, increasing the viscosity of suspensions, and high concentration of solutions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
I. Construction of CDNA Libraries
[0180] The BONTNOT01 cDNA library was constructed using RNA isolated from tibial periosteum removed from a 20-year-old Caucasian male during a hemipelvectomy with amputation above the knee. Pathology for the associated tumor tissue indicated partially necrotic and cystic osteoblastic grade 3 osteosarcoma, post-chemotherapy. Family history included osteogenesis imperfecta, closed fracture, and type II diabetes.
[0181] The LUNGNOT23 cDNA library was constructed using RNA isolated from left lobe lung tissue removed from a 58-year-old Caucasian male. Pathology for the associated tumor tissue indicated metastatic grade 3 (of 4) osteosarcoma. Patient history included soft tissue cancer, secondary cancer of the lung, prostate cancer, and an acute duodenal ulcer with hemorrhage. Family history included prostate cancer, breast cancer, and acute leukemia.
[0182] For construction of the BONTNOT01 and LUNGNOT23 cDNA libraries, frozen tissue from each of the above ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com