Method used for preparing Lopinavir using one-pot method
A technology of tetrahydropyrimidine and methylbutyric acid, applied in the field of medicine, can solve the problems of cumbersome operation and post-processing, increase the reaction steps of activated ester, and be unsuitable for industrial production, etc., and achieve easy separation and purification, short production cycle, synthesis and yield high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
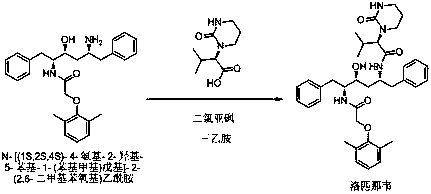
[0031] The present embodiment provides a method for preparing lopinavir by a one-pot method, specifically:
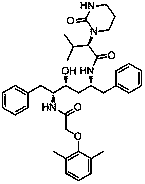
[0032] Add (2S)-(1-tetrahydropyrimidin-2-one)-3-methylbutanoic acid 20.0g (0.1mol) and dichloromethane 100ml into the reaction flask and place it under ice water, control the temperature below 10°C , 13.9 g (0.11 mol) of thionyl chloride was dropped into the reaction solution. After the dropwise addition was completed, the reaction was carried out at 0~10°C with stirring for 1 h, and then refluxed for 1 h to obtain (2S)-(1-tetrahydropyrimidine-2- Ketone)-3-methylbutyryl chloride reaction solution; the reaction solution was lowered to 0~20°C, 25.3g (0.25mol) of triethylamine was added, and placed under ice water, the temperature was controlled below 10°C, and N-[ (1S,2S,4S)-4-amino-2-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]-2-(2,6-dimethylphenoxy)acetamide 42.4 g (0.095mol) was added to the reaction solution, after the addition was completed, the reaction was carried ou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com