Lopinavir and ritonavir compound tablet and preparation method thereof
A technology of compound tablet and lopina, which is applied in the field of medicine, can solve the problems of unfavorable industrial production, complex preparation process, and high equipment requirements, and achieve the effect of uniform active ingredient content, simple operation, and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
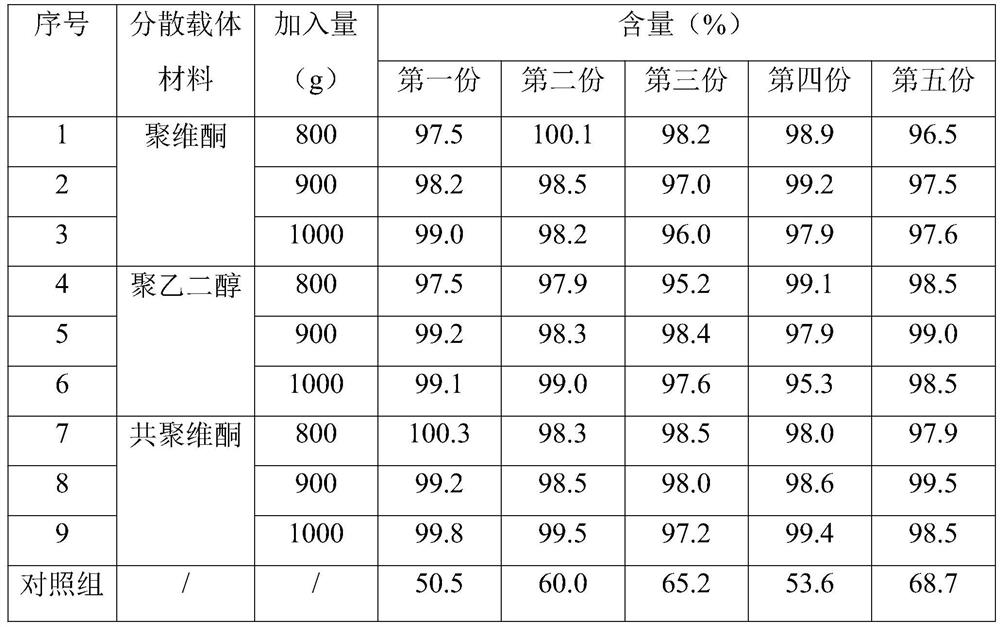
[0033] The present invention adopts the preparation method of hot-melt extrusion. When lopinavir and ritonavir prepare solid dispersions, it is necessary to mix the bulk drug and the dispersion carrier material, and the proportion of the dispersion carrier material is relatively large, and the uniformity of mixing It will affect the stability and uniformity of the product. In order to further improve the uniformity of mixing, the present invention further increases the uniformity of the finally prepared compound tablet through the selection and optimization of the dispersion carrier material.
[0034] As shown in Table 1, it is the screening process of partially dispersed carrier materials. Under the condition that the selection of each raw material in the prescription is the same and other parameters and conditions take intermediate values, the content uniformity of the prepared compound tablet is determined. Only the detection results of ritonavir are given in Table 1, and t...
experiment example 2
[0042]Since lopinavir and ritonavir are insoluble in water, it is necessary to investigate the dissolution rate of insoluble drugs. Usually, the method to improve the dissolution rate of poorly soluble drugs is to add disintegrants. Commonly used disintegrants include dry starch, sodium carboxymethyl starch, and cross-linked PVP. In the course of the invention, the applicant accidentally found that when the thickener was selected from the aqueous solution of hydroxypropyl methylcellulose E50, it was more preferably an aqueous solution of 5-20% (w / w) hydroxypropyl methylcellulose E50 When , the dissolution rate can be improved even without the addition of disintegrants. As shown in Table 3, it is the screening process of some viscosifiers and disintegrants (including different concentrations), and other parameters take intermediate values, and the prepared compound tablets are tested for dissolution. The dissolution rate is measured by the Chinese Pharmacopoeia dissolution tes...
Embodiment 1
[0047] raw material Content (g) Ritonavir 50 Lopinavir 200 Copovidone 639 polyethylene glycol 213 colloidal silica 10 Division 20 80
[0048] Ritonavir, lopinavir, copovidone, polyethylene glycol and colloidal silicon dioxide are mixed according to the above-mentioned content to prepare a mixed material, and the mixed material is pulverized until the particle diameter D90 is 150 μm and mixed; Set the extrusion temperature of the twin-screw hot-melt extruder to 100°C, start the screw after the temperature rises to the set value, and put the ground, pulverized and mixed material and Span 20 into the twin-screw hot-melt extruder , after melting and extruding, extruded in strips; crushed after being cooled by a cold roll, adding 50g of a tackifier, and the tackifier is 5% (w / w) of hydroxypropylmethylcellulose E50 The aqueous solution is passed through a 50-80 mesh sieve after drying, granulated by a shaking granulator and compress...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com