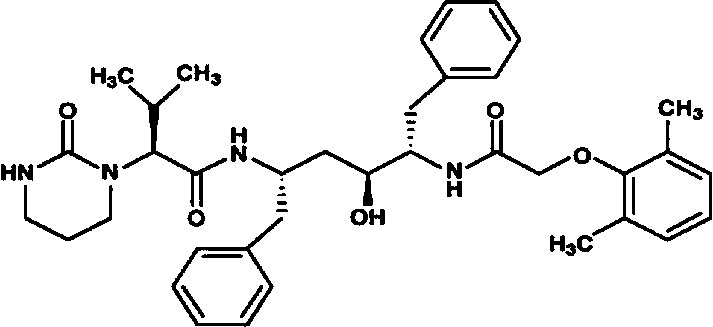
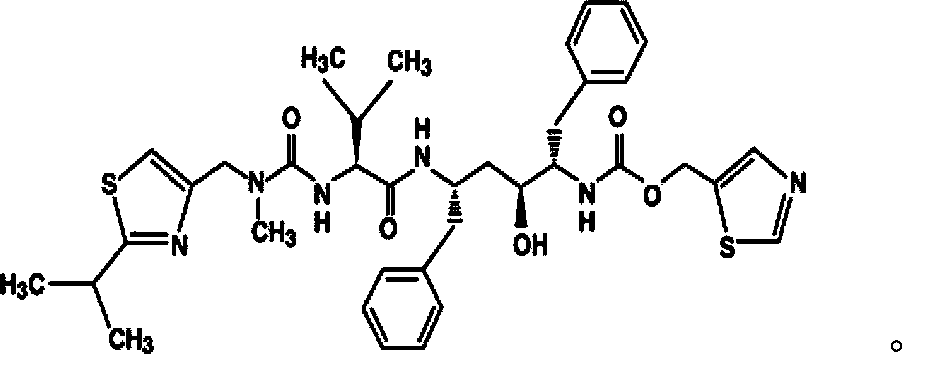
A kind of lopinavir and ritonavir compound high homogeneity nano co-dispersion and preparation method thereof
A technology of ritonavir and lopina, which is applied in the field of lopinavir and ritonavir compound high-uniformity nano-co-dispersion and its preparation, and can solve the problems of heavy tablets, inconvenience in taking, and temperature control. Uniformity is not very good and other problems, to achieve the effect of simple and easy preparation method and great application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] formula:
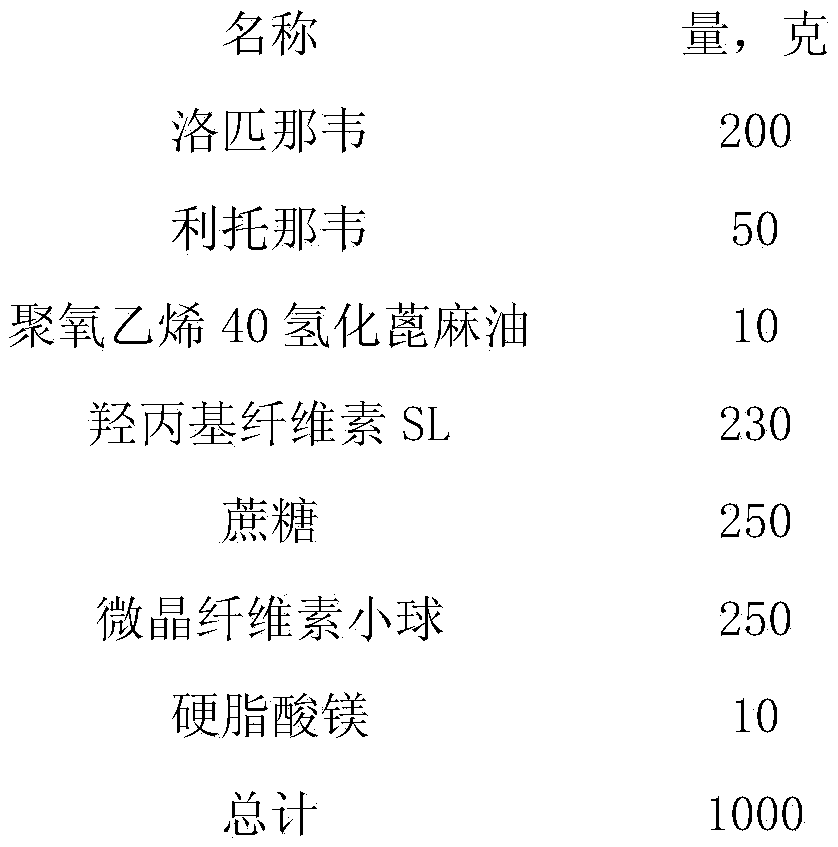
[0048] name
Quantity (grams)
200
50
Polyoxyethylene 40 Hydrogenated Castor Oil
10
230
250
microcrystalline cellulose pellets
250
10
total
1000
[0049] preparation:
[0050] Disperse 200 grams of lopinavir, 50 grams of ritonavir and 10 grams of polyoxyethylene 40 hydrogenated castor oil in 1800 grams of purified water, add 230 grams of hydroxypropyl cellulose into the above dispersion, stir and dissolve, and disperse evenly. The dispersion is ground using a nano-grinding machine, monitored while grinding, and ground for 3.5 hours. The particle size distribution is below 400 nm, and the D90 is 312 nm. After the grinding is completed, a stock solution is prepared; 250 grams of sucrose is dissolved in 1160 grams of purified water, After the dissolution is complete, slowly pour it i...
Embodiment 2
[0052] formula:
[0053] name
Quantity (grams)
100
25
Laurel Sorbitan
50
Hydroxypropyl Methyl Cellulose E3
25
Sodium carboxymethyl cellulose
12
25
13
1000
total
1250
[0054] preparation:
[0055] Evenly disperse 100 grams of lopinavir, 25 grams of ritonavir and 50 grams of sorbitan laurel in 1000 grams of purified water, add 25 grams of hydroxypropyl methylcellulose into the above dispersion, stir and dissolve, and disperse evenly. The dispersion is ground using a nano-grinding machine, monitored while grinding, and ground for 5.3 hours, and the particle size distribution reaches below 250 nanometers, and D90 is 210 nanometers. After grinding, it is prepared into stock solution one; 1 g of microcrystalline cellulose and 13 g of talc powder were added to stock solution 1, and circulat...
Embodiment 3
[0057] formula:
[0058] name
Quantity (grams)
100
25
Polysorbate 80
10
30
Sodium carboxymethyl cellulose
25
[0059] Microcrystalline Cellulose RC591
50
25
purified water
400
total
665
[0060] preparation:
[0061] Evenly disperse 100 grams of lopinavir, 25 grams of ritonavir and 10 grams of polysorbate 80 in 400 grams of purified water, add 30 grams of hydroxypropyl cellulose into the above dispersion, stir and dissolve, and disperse evenly. Grind with a nano grinder, monitor while grinding, and when grinding for 5.5 hours, the particle size distribution reaches below 250 nanometers, and D90 is 221 nanometers. After grinding, stock solution 1 is prepared; 25 grams of sodium carboxymethyl cellulose, 50 grams Microcrystalline cellulose and 25 grams of talcum powder were added to the stock...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com