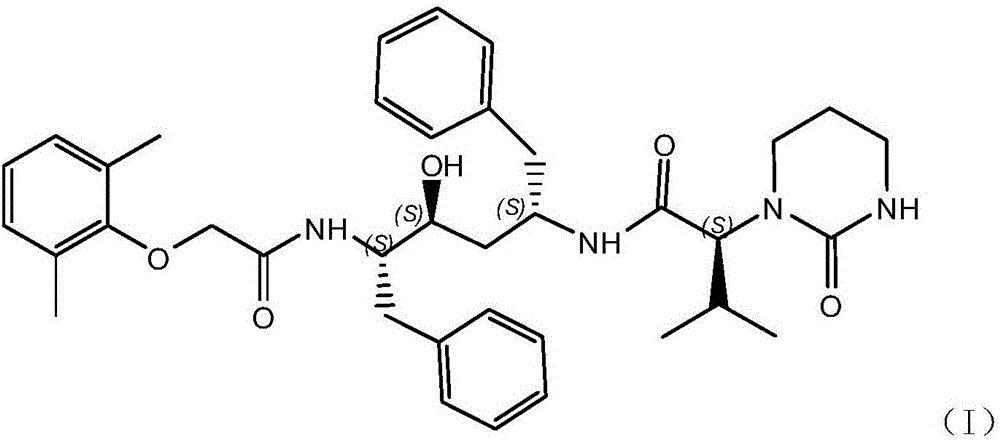
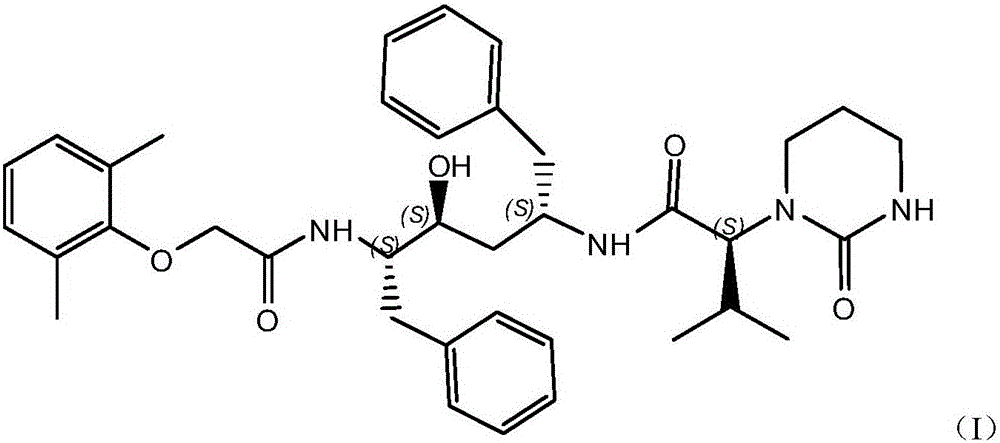
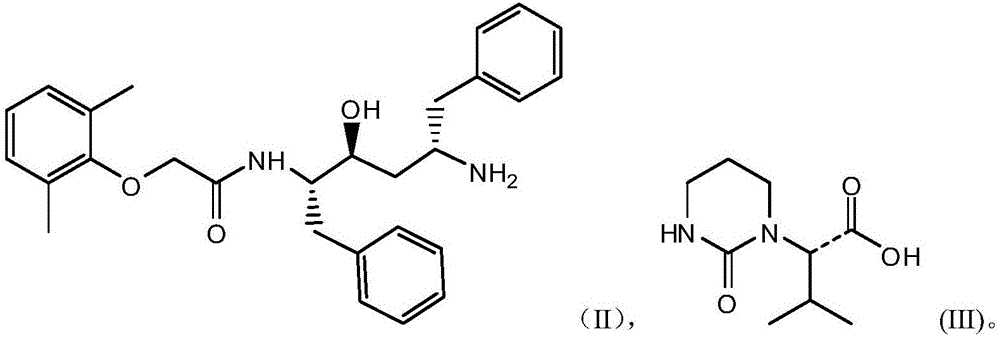
Lopinavir preparation and purification process
A lopinavir and process technology, applied in the field of drug synthesis, can solve the problems that lopinavir is difficult to meet the USP standard requirements and cannot remove impurities, and achieve the effect of low cost and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation method of embodiment 1 lopinavir crude product
[0026] Put 147kg of dimethylformamide into the reaction kettle, put in 20kg of the compound shown in formula (II), stir to completely dissolve, put in 9kg of compound shown in formula (III), fill it with nitrogen and cool down to 0°C to strengthen nitrogen protection, put in 18.2kg of HOBT, After stirring for complete dissolution, add DCC solution (DCC18.6kg / dimethylformamide 8kg), add triethylamine 9.2kg, control the internal temperature at 25°C, keep stirring and react for 12 hours, and follow the reaction end point by HPLC. (The raw material does not have a peak or the proportion of the peak area is less than 0.05%, and the next step can be processed). After the reaction is complete, control the internal temperature to about 25°C, add 200kg of drinking water, 200kg of ethyl acetate, control the internal temperature to 25°C, and stir After 30 minutes, the organic layer was left to stand for layering, and...
Embodiment 2
[0029] The purification process of embodiment 2 lopinavir crude product
[0030] Put 20kg of crude lopinavir into 310kg of methanol and stir for 60 minutes to confirm complete dissolution, stir and cool down to 0-5°C, after complete dissolution, cool down to 0-5°C, add 179kg of water, continue to cool down to -10°C after adding water ℃, heat preservation and stirring for 10-30 minutes, (there will be yellow oil on the reaction vessel), let stand for 10 minutes, separate the supernatant, slowly drop 8kg of water into the supernatant and stir until the solid precipitates, confirm that the solid precipitates before Add 200kg of water (20+40+60+80) in batches and add once every 10 minutes. After the addition is complete, keep stirring at 10-15°C for 1 hour, shake off the centrifuge, put the wet product into a hot air oven, and control the internal temperature to 50 Dry at ~55°C for 48 hours to obtain 18.2kg, and perform high-performance liquid phase HPLC detection according to the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com