Oral suspension preparation containing lopinavir and ritonavir
A technology of lopinavir and ritonavir, which is applied in the field of compound oral suspension containing lopinavir and ritonavir and its preparation, can solve the problem of drug stability, complex drug components, and technical problems. Complicated and other issues, to achieve good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] The following examples describe the technical solutions of the present invention in detail, but the present invention is not limited only to the examples.
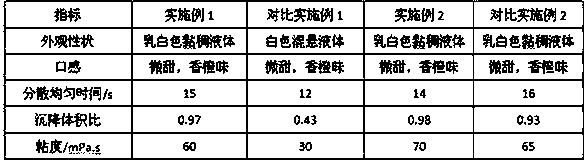
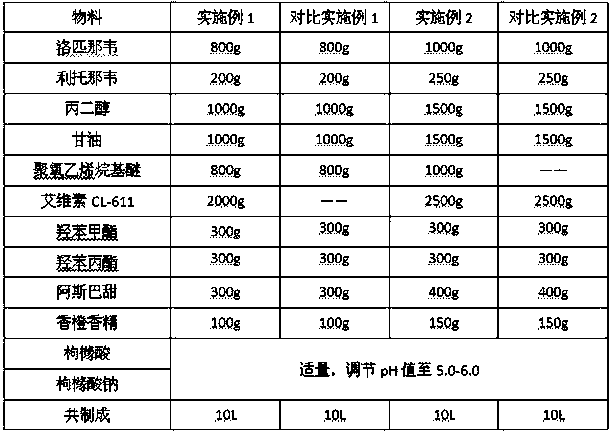
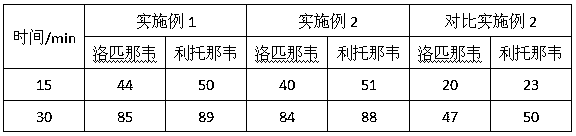
[0024] Prescription composition:
[0025]
[0026] Preparation Process:
[0027] (1) Heat glycerin and propylene glycol to 50-55°C, add methylparaben and propylparaben, and dissolve to make a preservative solution;
[0028] (2) Add Avicel CL-611 (not added in Comparative Example 1) into water, and use high-shear equipment to disperse it evenly and present a uniform suspension system;
[0029] (3) Mix purified water heated to 50-55°C with polyoxyethylene alkyl ether (not added in Comparative Example 2) to prepare a dispersion;
[0030] (4) Mix aspartame, orange essence, the preservative solution prepared in step (1) and the suspension system obtained in step (2) evenly, and set aside;
[0031] (5) Mix the micronized lopinavir and ritonavir with the dispersion in step (3) evenly, add to the mixture obtained in s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com