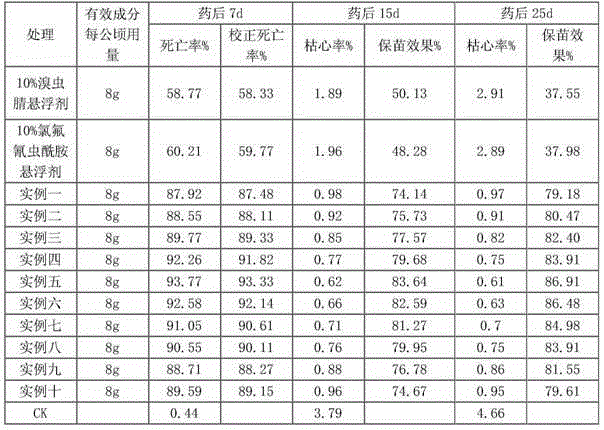
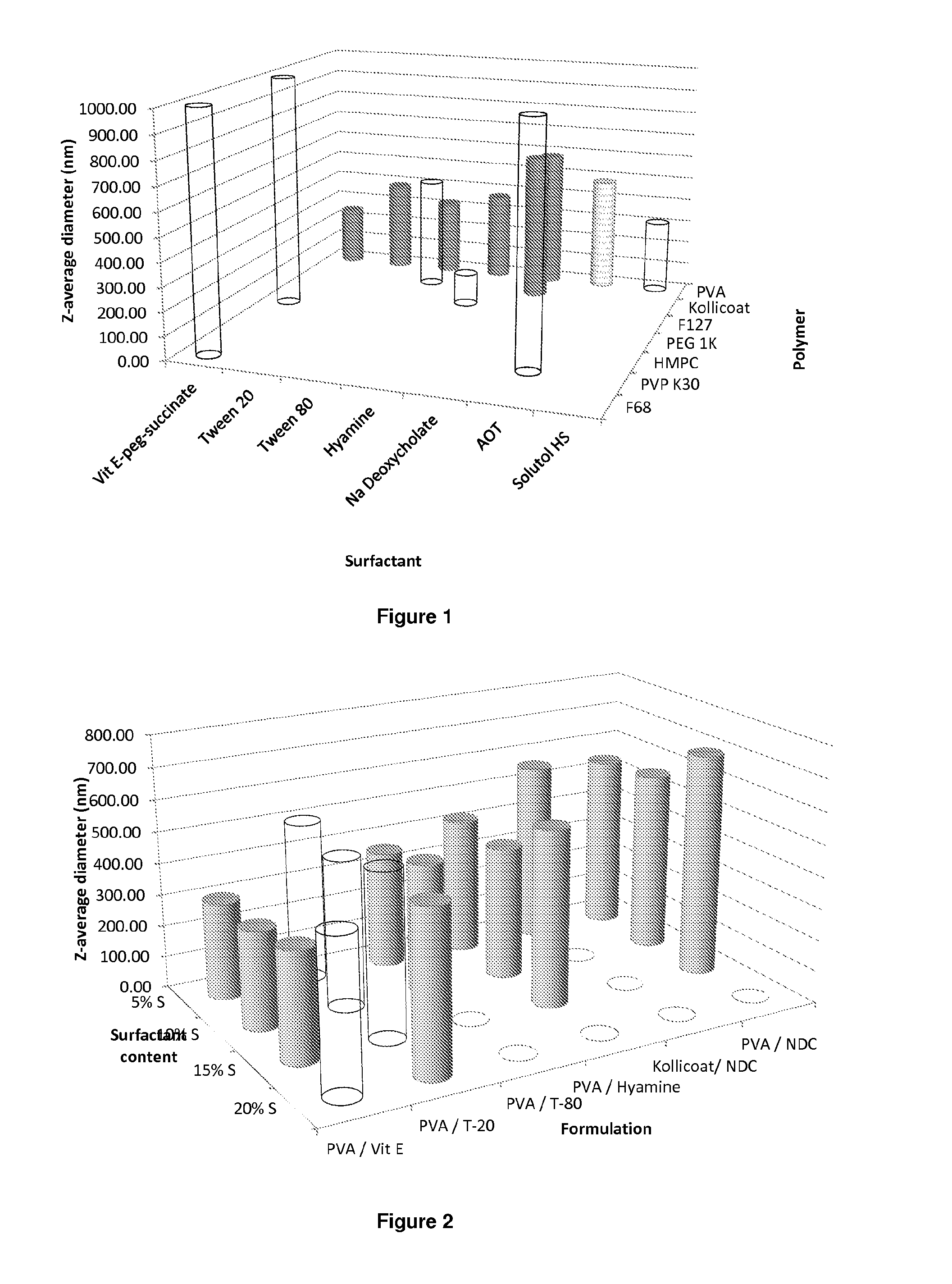
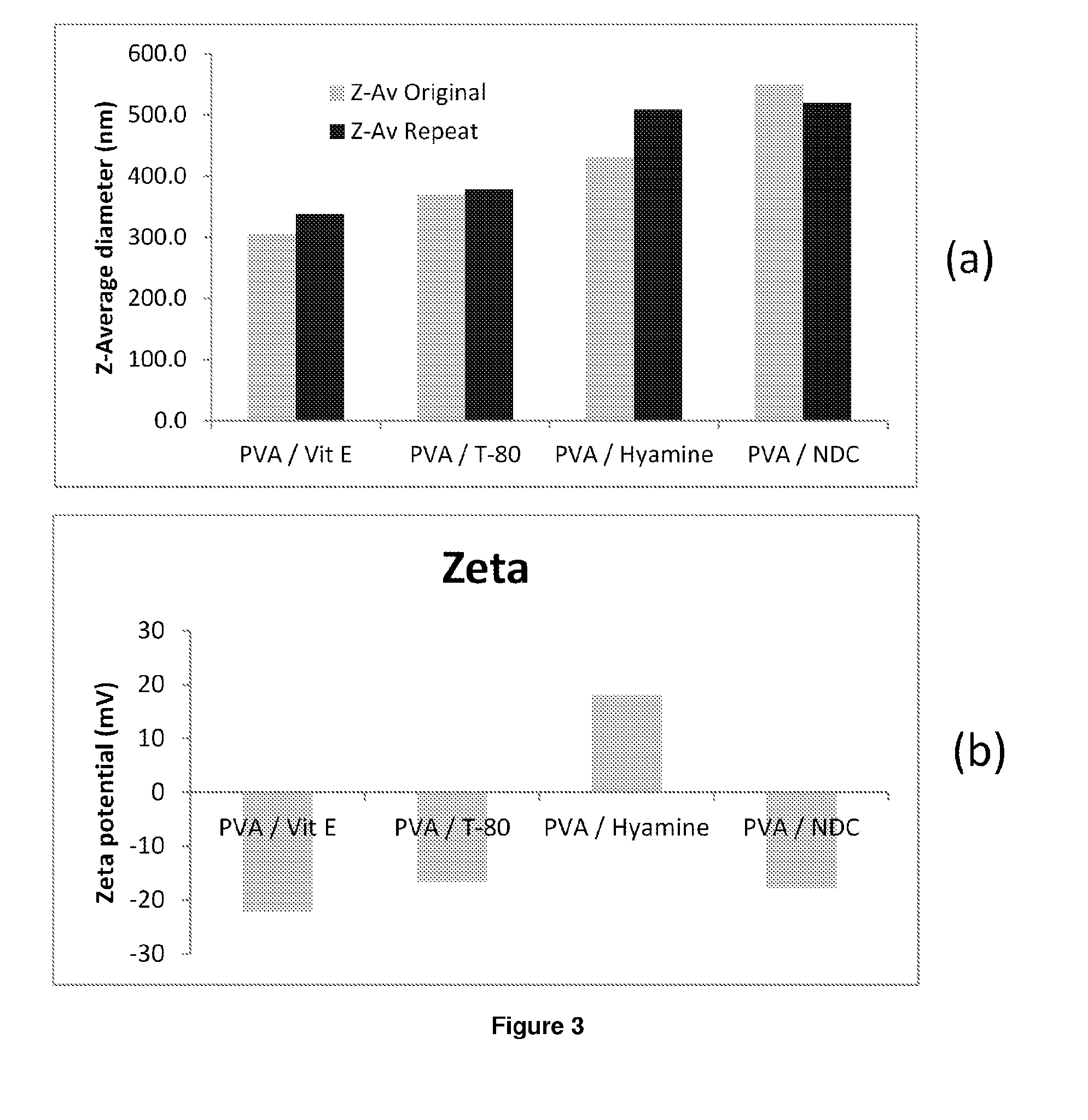
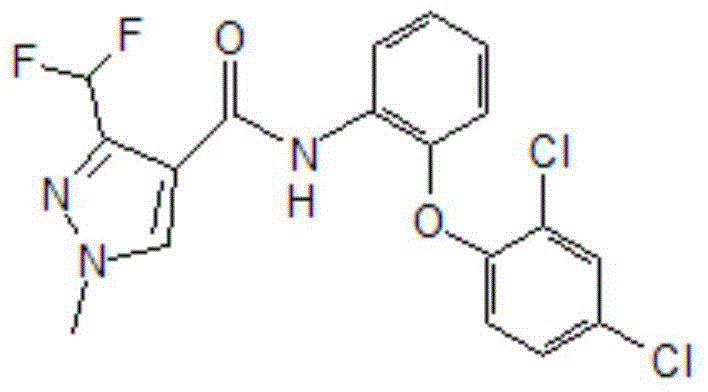
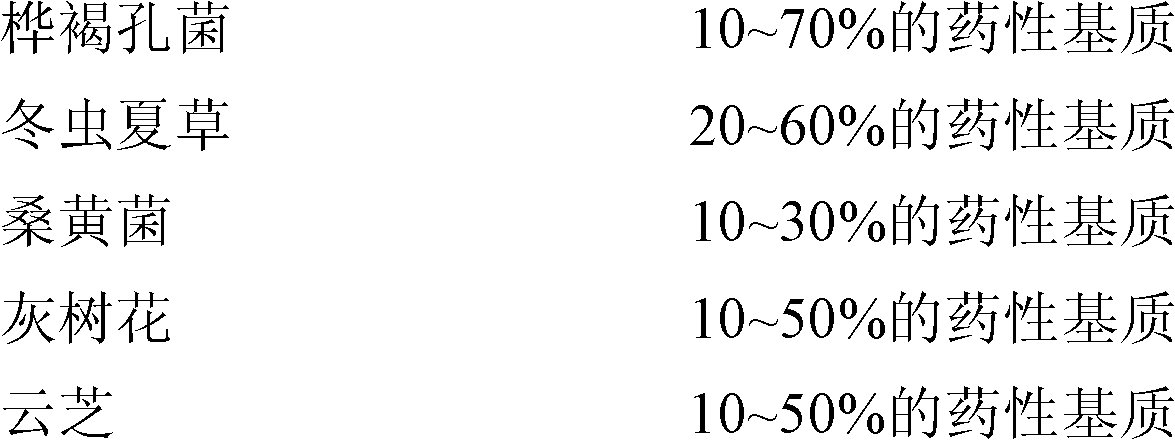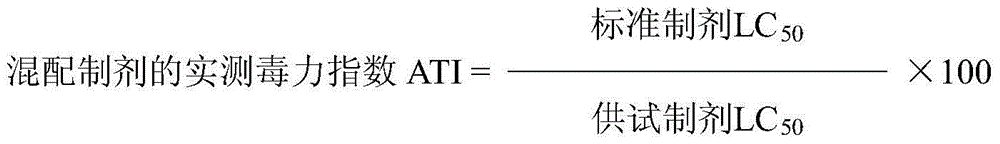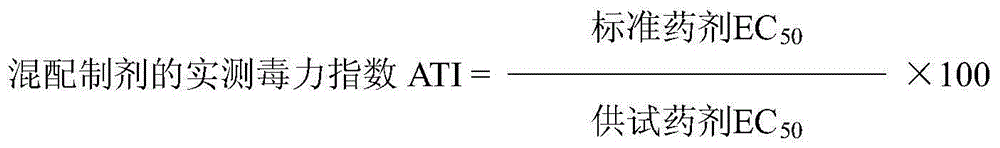Patents
Literature
98results about How to "Advanced dosage form" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Colonic purgative composition with soluble binding agent
ActiveUS20050129781A1Improve dosage form characteristicSimple preparation processBiocideInorganic phosphorous active ingredientsBowel cleansingTolerability
This invention relates to novel colonic purgative compositions in a solid dosage form, comprising at least one purgative and at least one soluble, or soluble, nonfermentable binder, such as polyethylene glycol. Further, this invention relates to methods of using the colonic purgative compositions. The present compositions and methods are designed to improve patient tolerance and compliance, while at the same time improving the quality of bowel cleansing. The formulations and methods of this invention are particularly useful to cleanse the bowel prior to diagnostic and surgical procedures and can also be employed in lower dosages as a laxative to promote elimination and / or to relieve constipation.
Owner:SALIX PHARMA INC
Fungal pharmaceutical mycoplasm with blood sugar lowering efficacy and preparation method thereof
InactiveCN102428832AAvoid secondary damageEnsure safetyAnthropod material medical ingredientsMetabolism disorderPhellinus igniariusInonotus obliquus
The invention belongs to the field of biofermentation engineering. A pharmaceutical medium and a fungal strain which have blood sugar lowering efficacy are used as bidirectional fermentation raw materials, wherein the pharmaceutical medium is mainly composed of the following raw materials: cornel, gynostemma, momordica grosvenori, winged euony twigs, balsam pear and polyrhachis vicina; the fungalstrain is composed of the following strains: inonotus obliquus, cordyceps, phellinus igniarius, polystictus versicolor, and grifola frondosa; and bi-directional multi-fungi fermentation is carried out between multiple edible and pharmaceutical fungi and Chinese herbal medicines by a bidirectional fermentation process, thus organisms in two kingdoms or three kingdoms are organically combined to obtain an entirely new blood-sugar-lowering pharmaceutical mycoplasm product. The obtained product can generate 1+1>2 physiological function efficiency for hypoimmunity, has the actions of obviously lowering blood sugar but not increasing insulin concentration, has significant positive effects on helping treating and controlling diabetic complications, and can be used as an oral medicament for preventing and treating diabetes or a health-care product for adjusting blood sugar.
Owner:DALIAN BAIXIANGJU BIOLOGICAL TECH
Benzoic acid, benzoic acid derivatives and heteroaryl carboxylic acid conjugates of hydromorphone, prodrugs, methods of making and use thereof
ActiveUS8816083B2Lower potentialReduced drug abuse potentialBiocideNervous disorderBenzoic acidEpoxy
The presently described technology provides compositions comprising aryl carboxylic acids chemically conjugated to hydromorphone (4,5-α-epoxy-3-hydroxy-17-methyl morphinan-6-one) to form novel prodrugs / compositions of hydromorphone. The hydromorphone prodrugs of the present technology have decreased side effects and decreased potential for abuse compared to unconjugated hydromorphone. The present technology also provides methods of treating patients, pharmaceutical kits and methods of synthesizing conjugates of the present technology.
Owner:KEMPHARM INC
Compositions of lopinavir and ritonavir
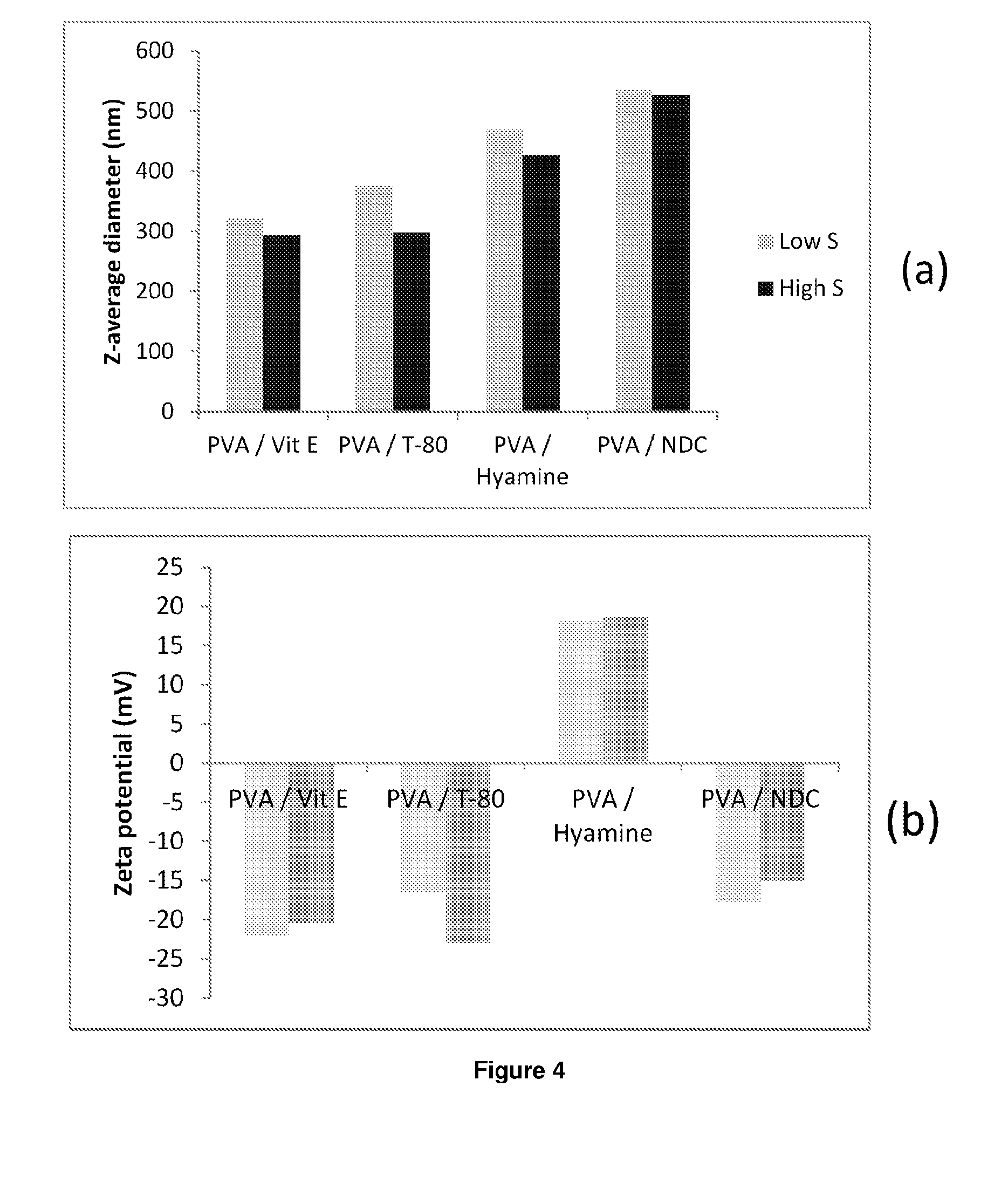
ActiveUS20140220141A1High drug loadingOptimize allocationPowder deliveryBiocidePVA - Polyvinyl alcoholNanoparticle
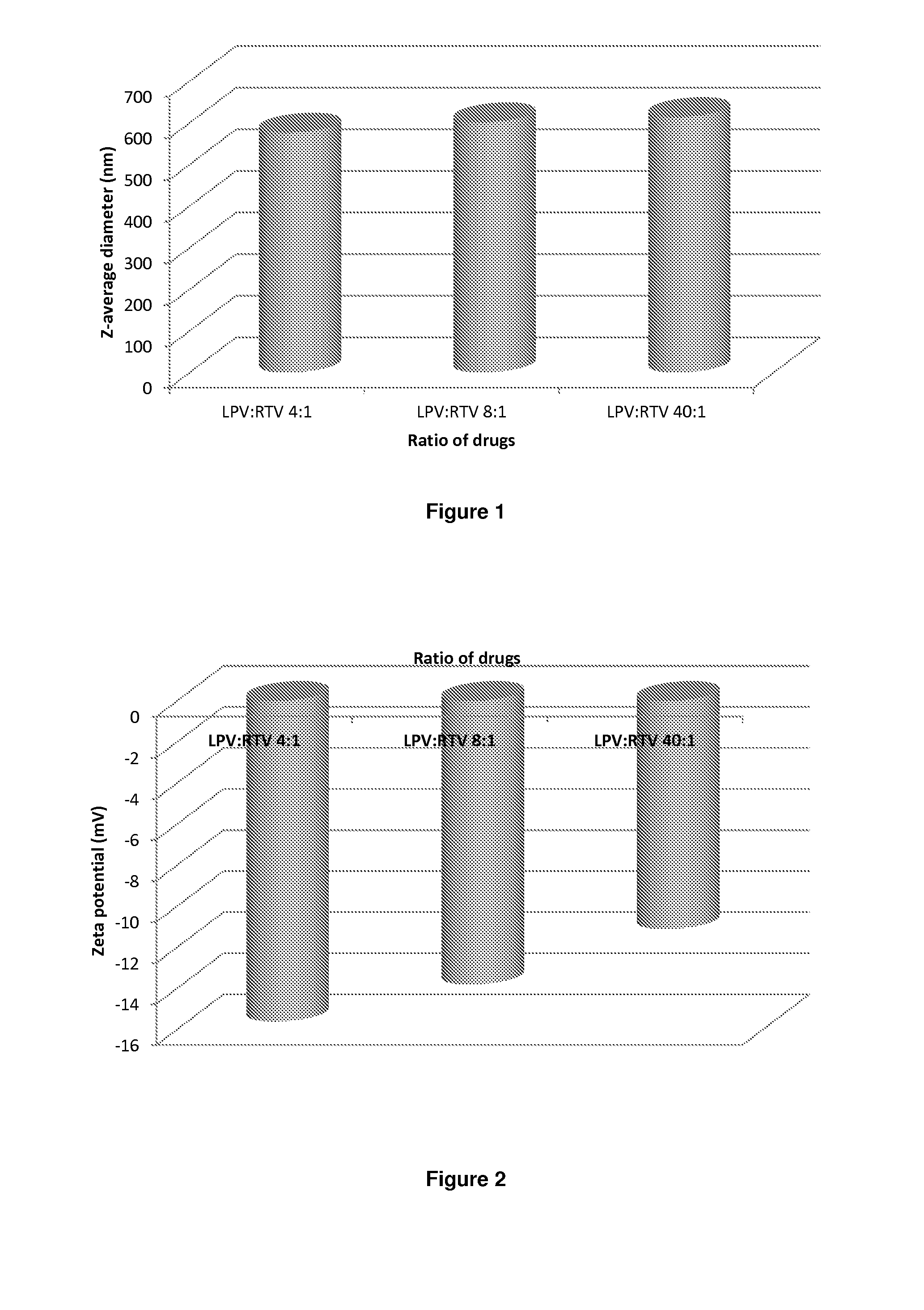
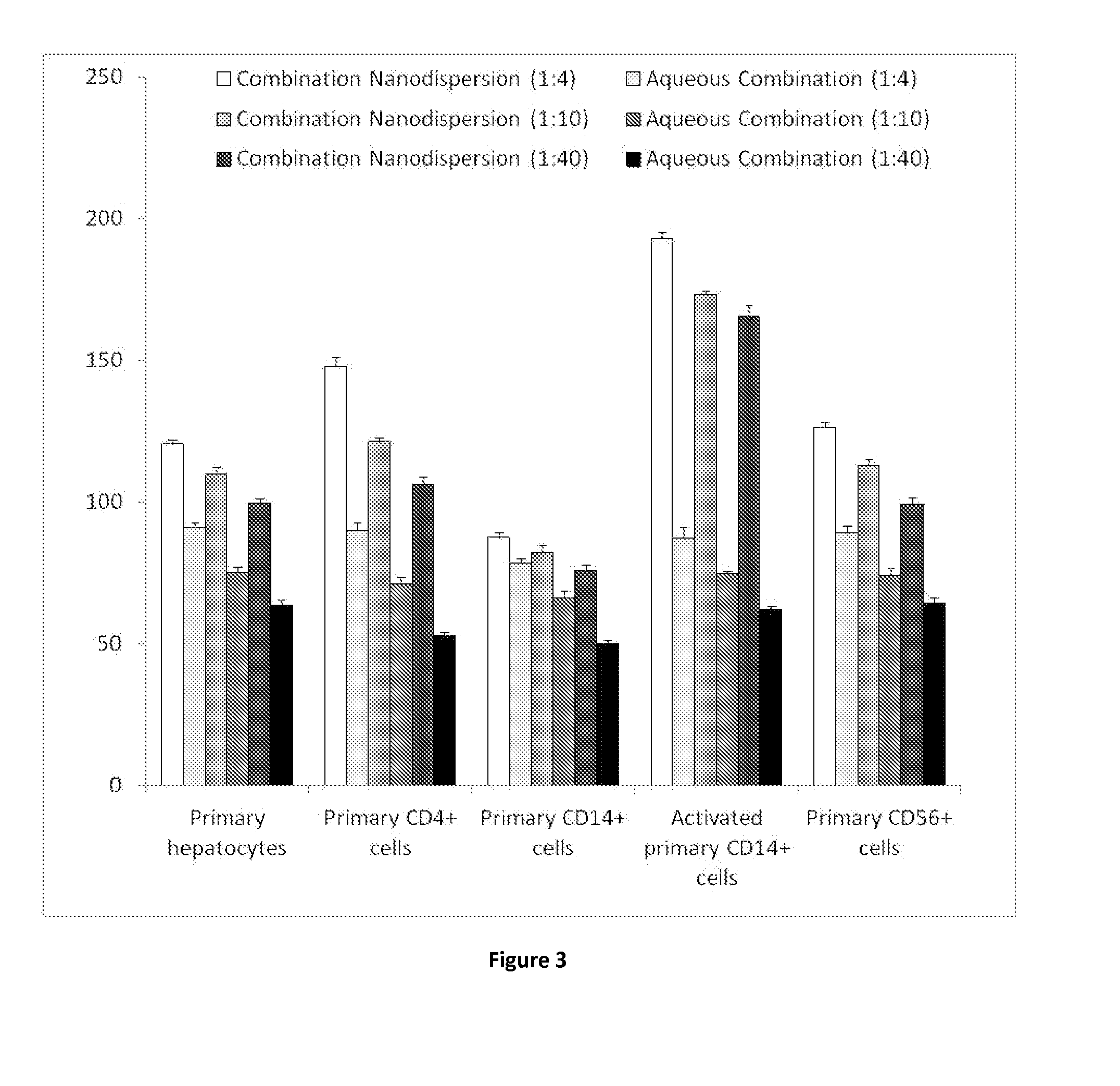
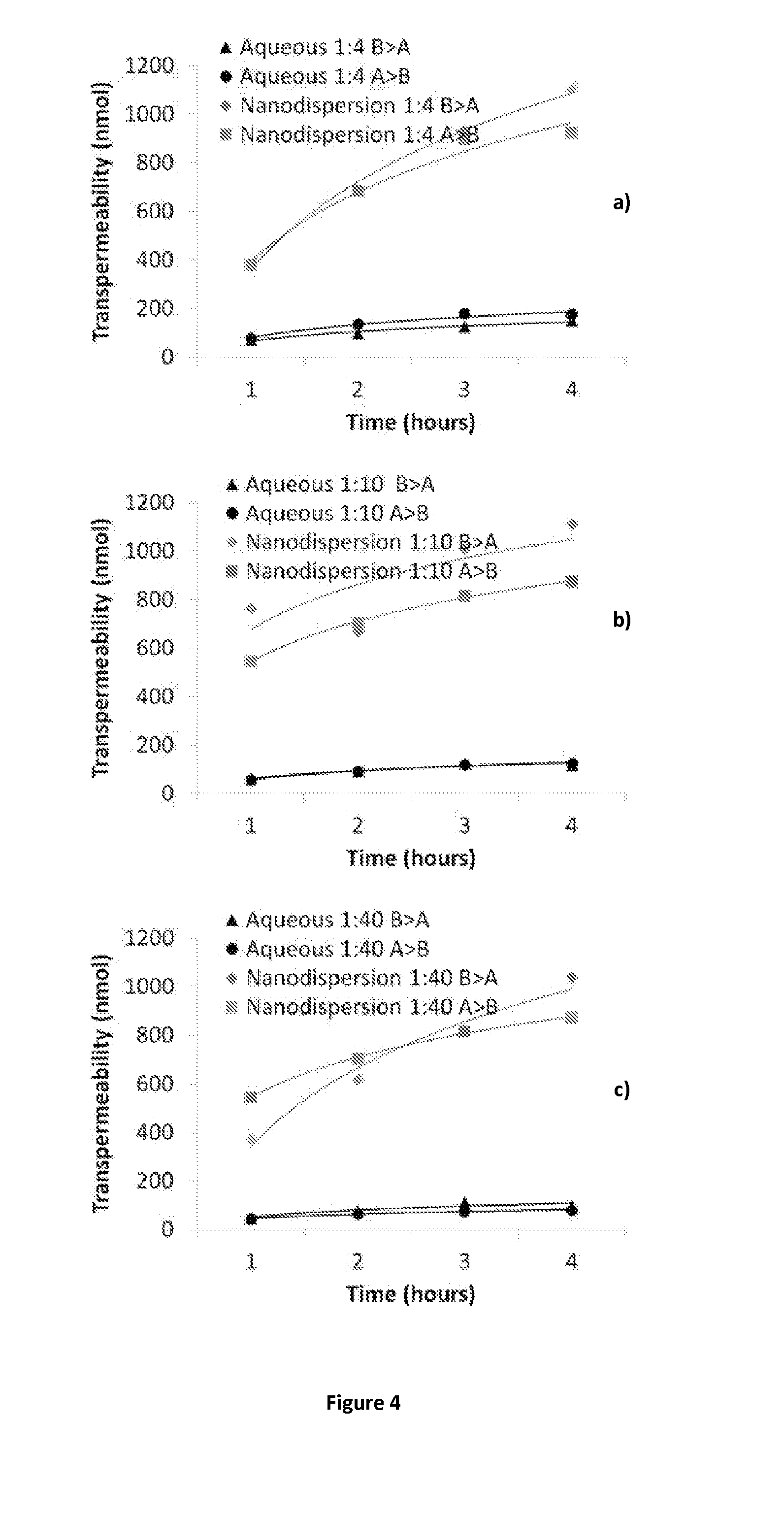
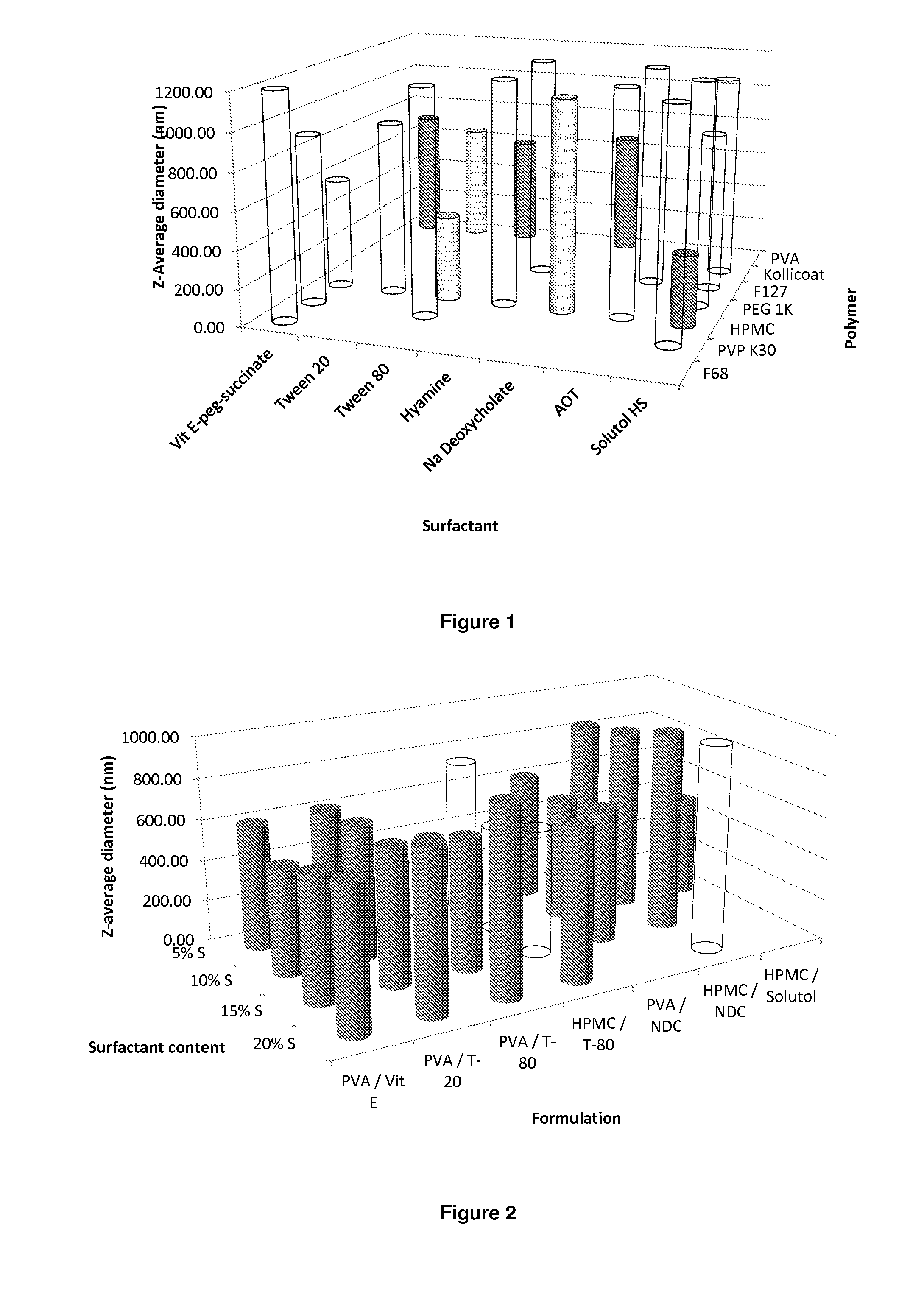
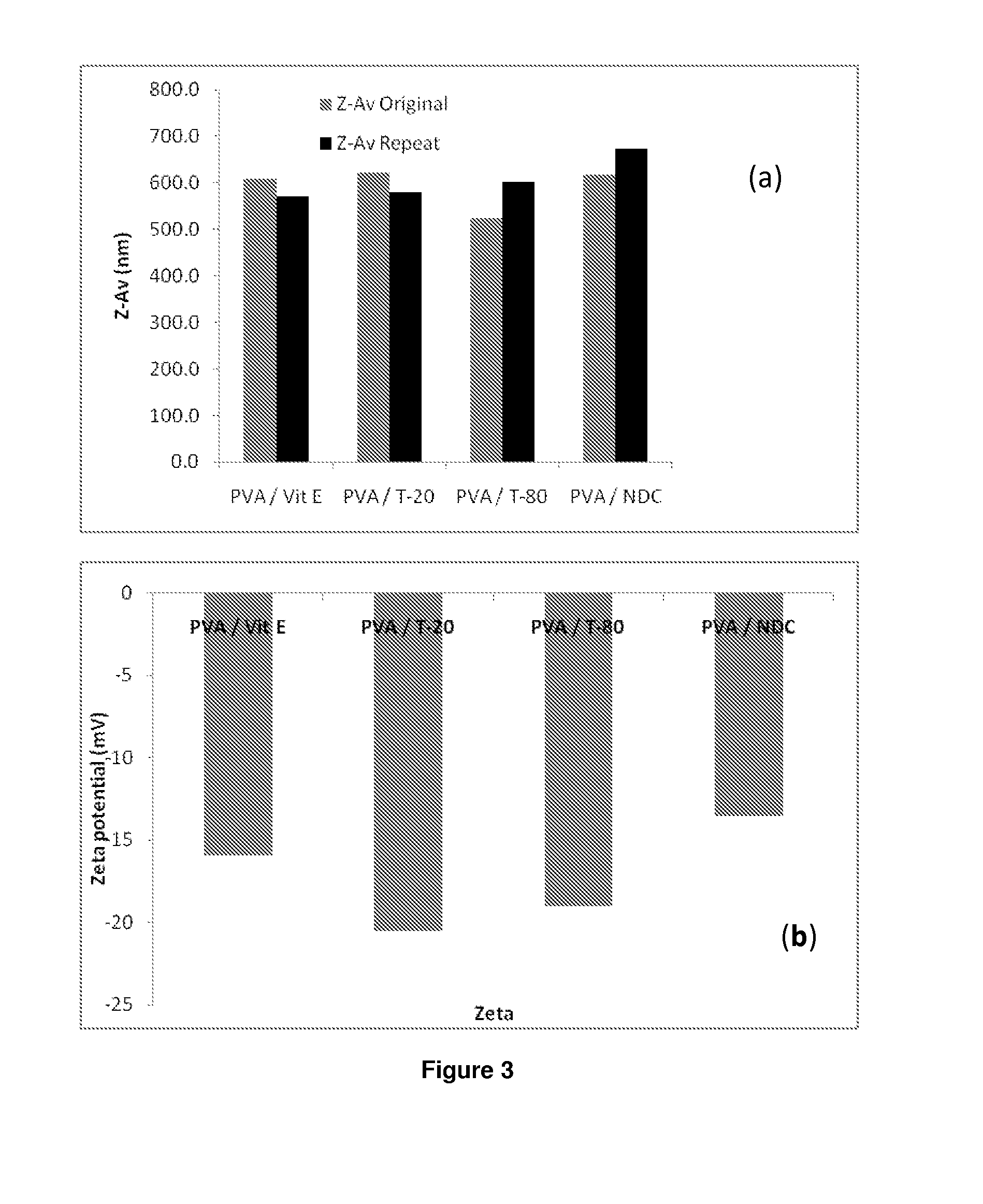
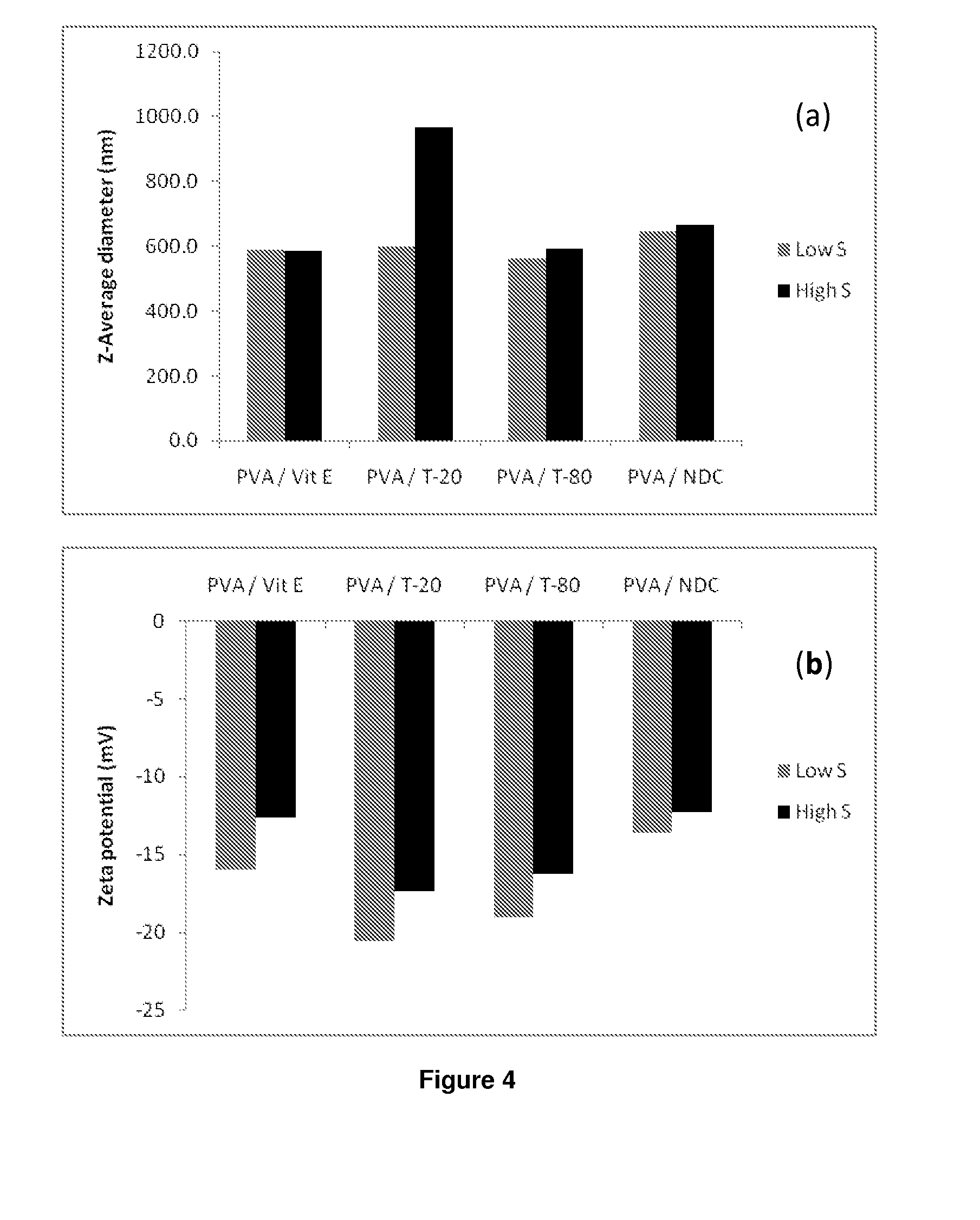
The present inventions relates to a solid composition and an aqueous dispersion comprising nanoparticles of the anti-retroviral drugs lopinavir and ritonavir. The solid composition and aqueous dispersion additionally comprise a mixture of a hydrophilic polymer and a surfactant. The surfactant is selected from vitamin-E-polyethylene glycol-succinate (Vit-E-PEG-succinate), a polyoxyethylene sorbitan fatty acid ester, N-alkyldimethylbenzylammonium chloride, sodium deoxycholate, dioctyl sodium sulfosuccinate, polyethyleneglycol-12-hydroxystearate, polyvinyl alcohol (PVA), and a block copolymer of polyoxyethylene and polyoxypropylene, or a combination thereof. The hydrophilic polymer is suitably selected from polyvinyl alcohol (PVA), a polyvinyl alcohol-polyethylene glycol graft copolymer, a block copolymer of polyoxyethylene and polyoxypropylene, polyethylene glycol, hydroxypropyl methyl cellulose (HPMC), and polyvinylpyrrolidone, or a combination thereof. The present invention also relates to processes for preparing both the solid composition and the aqueous dispersion, as well as to their use in therapy for the treatment and / or prevention of retroviral infections such as human immunodeficiency virus (HIV).
Owner:UNIV OF LIVERPOOL
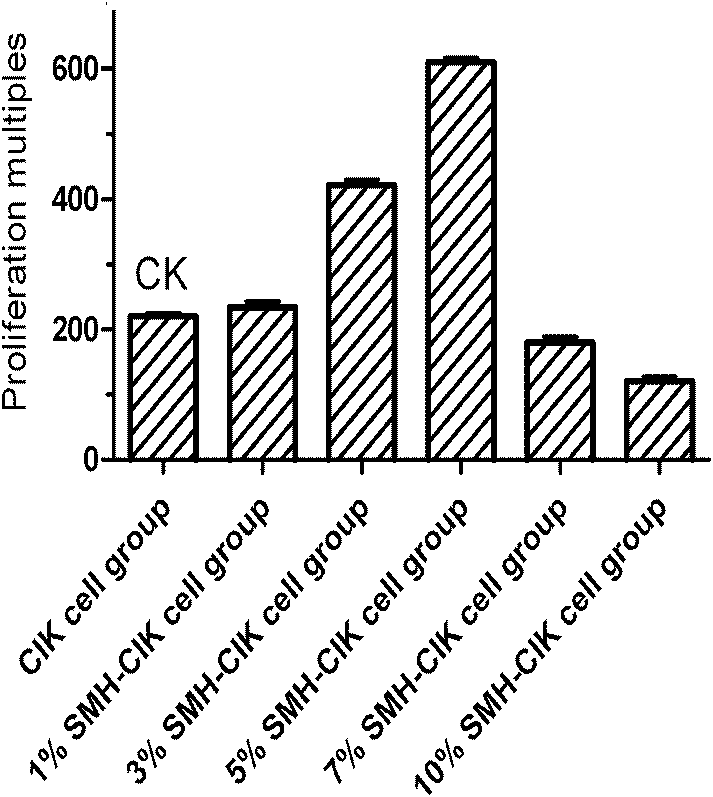
Traditional Chinese medicine extract capable of improving CIK cell proliferation rate as well as preparation method and application of same
InactiveCN102755512APrescription scouringQuality is easy to controlImmunological disordersAntineoplastic agentsBiotechnologyFormulary
The invention relates to a traditional Chinese medicine extract capable of obviously improving CIK (Cytokine-Induced Killer) cell proliferation rate. The extract is prepared from the raw materials of red ginseng, radix ophiopogonis and milk vetch according to a certain weight ratio. A formula of the extract comprises the steps as follows: the red ginseng, the radix ophiopogonis and the milk vetchare added with water and decocted, and are subjected to ethanol precipitation and filtered; ethanol is recovered; the extract liquid is enriched with macroporous resin columns, eluted to be colorlesswith distilled water and eluted with ethanol; the eluant is collected; the extract liquid is filtered with a microporous filtration film; and the filter liquor is condensed, the pH value is adjusted,the filter liquor is filtered again with the microporous filtration film, sterilized, and subpackaged, and then the traditional Chinese medicine extract is obtained. Pharmacological experiments show that the traditional Chinese medicine extract can remarkably improve the proliferation rate of CIK cells.
Owner:CHINA JILIANG UNIV
Medicine for treating child diarrhea and its preparation method
ActiveCN1559528AEasy to takeTake it accuratelyDigestive systemUnknown materialsSodium bicarbonateOfficinal
A Chinese medicine in the form of effervescent tablet for treating infantal diarrhea is prepared from 7 Chinese-medicinal materials including pilose asiabell root, pueraria root, liquorice root, etc and 3 medicinal additives: starch, dicarbonate and citric acid.
Owner:JIANGSU KANION PHARMA CO LTD
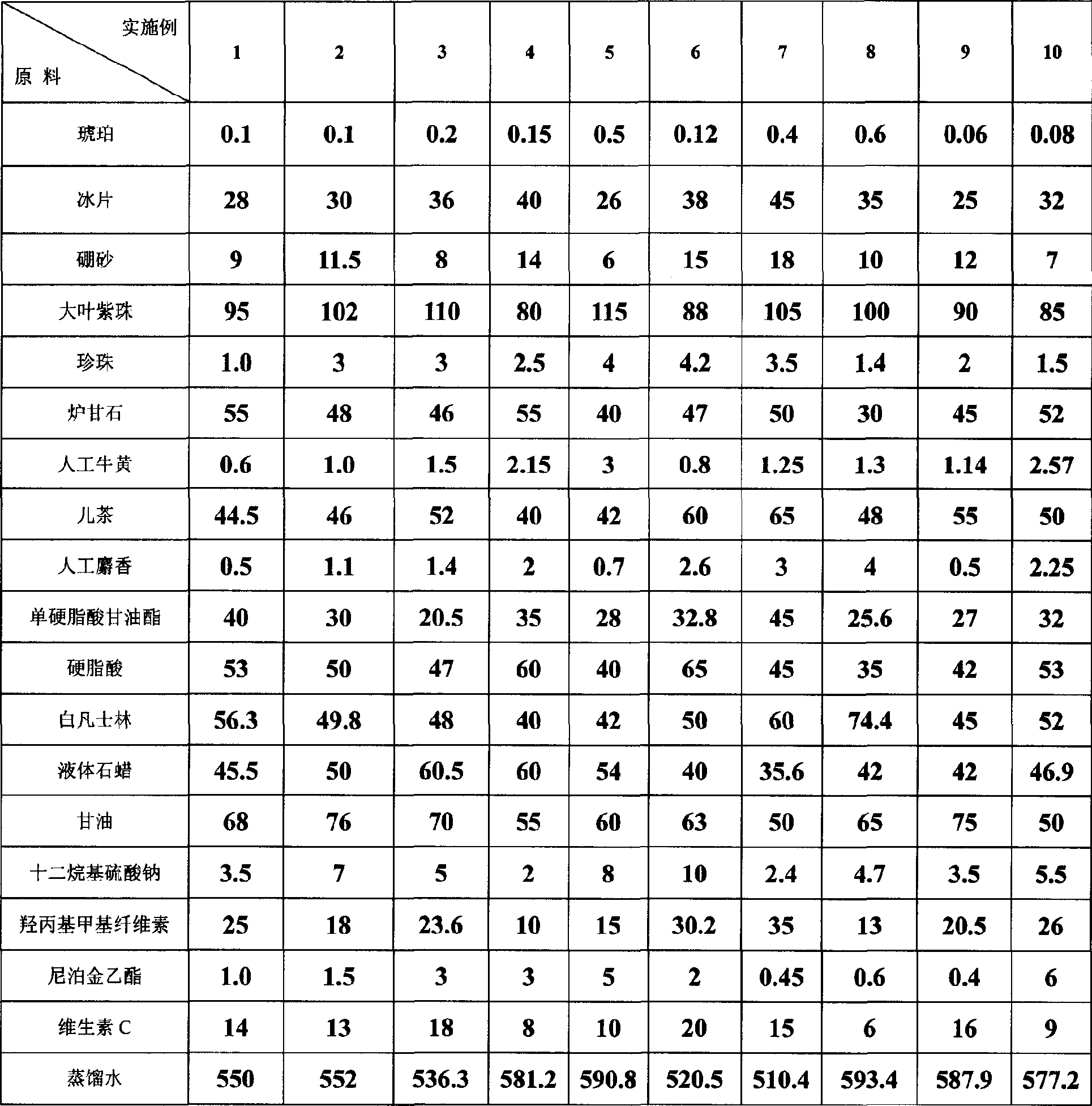
Gel for treating hemorrhoidal anus and rectum diseases, and its prepn. method
ActiveCN1903289ASmall particle sizeHigh yieldInorganic boron active ingredientsHydroxy compound active ingredientsDiseaseMixed haemorrhoid
A medicine in the form of gel for treating internal pile, external pile, mixed pile and fissure in gelling agent is prepared from 9 Chinese-medicinal materials including amber, borneol, borax, pearl, etc, 9 Chemicals including monoglyceride stearate, white vaseline, glycerin, VC, etc, and distilled water. Its preparing process is also disclosed.
Owner:MAYINGLONG PHARMA GROUP
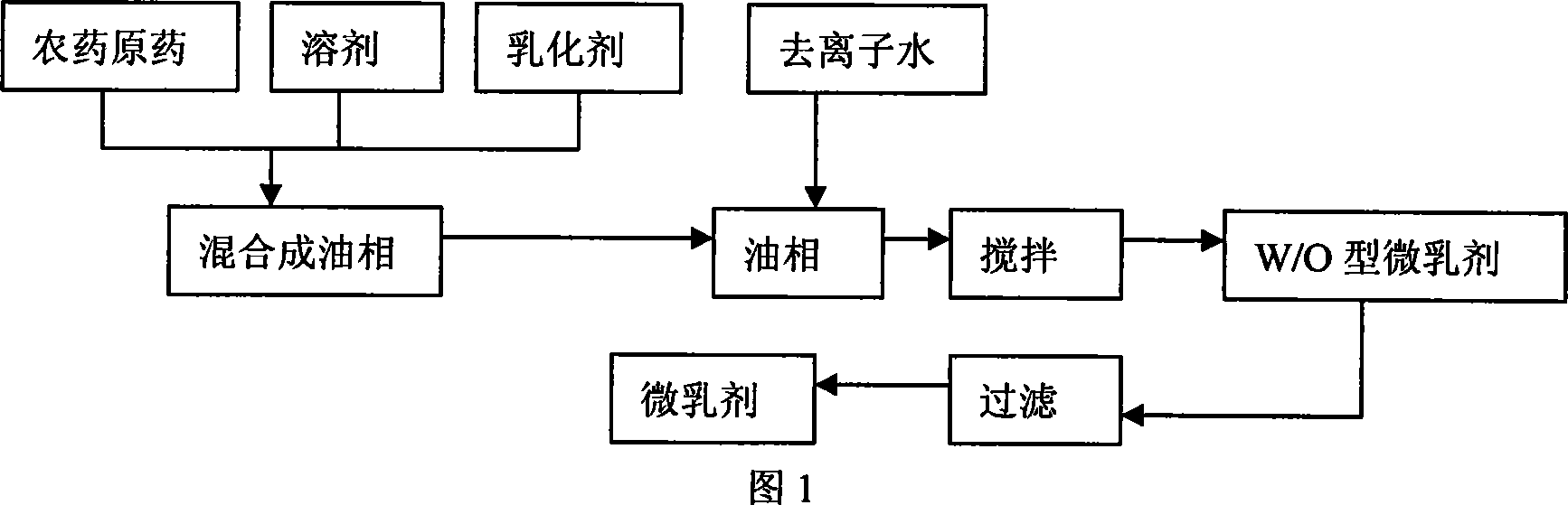
Ginkgo snail-killing micro emulsion and preparation method thereof
The invention discloses a gingko snail killing microemulsion agent and a preparing method, which relates to the technical field of plant source agricultural drug production. The invention takes the seed shell extract of the gingko containing a ginkgoic acid as the source drug, and the invention is made by configuring auxiliary surface active agents, solution agent and antifreeze agent as well as water. The producing method comprises the following operating steps: the gingko external seed shell extract, the microemulsion agent and the solution agent are blended to be an even and transparent oil phase; the steamed water or the deionized water are added slowly to form an oil enclosed water type emulsion during the agitating process; through agitating and heating, the emulsion is rapidly converted into a water enclosed oil type; a steady O or W type emulsion after being cooled to reach the constant indoor temperature is obtained. The invention has fine environmental compatibility of the emulsion conformation, also completely realizes the advantages of the gingko snail killing agent with high efficiency to the snail and safety to people and animals. The microemulsion agent takes water as the main solvent with slight influence to environment. In addition, the invention has remarkable synergism; the LC 50 and LC 90 of the 24h respectively are 0.56mg per liter and 3.5mg per liter, which are obviously better than that of the 24h snail killing effect (LC50 is equal to 1.35mg per liter and the LC90 is equal to 3.85mg per liter) of the external seed shell extract of the original drug.
Owner:JIANGSU UNIV
Water-based epoxy asphalt waterproof adhesive layer material and preparation method thereof
ActiveCN105925244AAdvanced dosage formGood surface permeabilityNon-macromolecular adhesive additivesPolyureas/polyurethane adhesivesEpoxyWater based
The invention discloses a water-based epoxy asphalt waterproof adhesive layer material and a preparation method thereof. The water-based epoxy asphalt waterproof adhesive layer material is prepared by adding expanded vermiculite powder and graphene on the basis of the traditional material, thereby greatly enhancing the impact resistance, strength and toughness of the material. The water-based epoxy asphalt waterproof adhesive layer material has favorable flame-retardant and smoke inhibition effects, so that the service life of the material is greatly prolonged; and thus, the water-based epoxy asphalt waterproof adhesive layer material is a low-cost high-performance novel waterproof adhesive layer material.
Owner:江苏金苏泽工程技术有限公司
Colonic purgative composition with soluble binding agent
ActiveUS20080213393A1Advanced dosage formSimple preparation processBiocideInorganic phosphorous active ingredientsBowel cleansingTolerability
This invention relates to novel colonic purgative compositions in a solid dosage form, comprising at least one purgative and at least one soluble, or soluble, nonfermentable binder, such as polyethylene glycol. Further, this invention relates to methods of using the colonic purgative compositions. The present compositions and methods are designed to improve patient tolerance and compliance, while at the same time improving the quality of bowel cleansing. The formulations and methods of this invention are particularly useful to cleanse the bowel prior to diagnostic and surgical procedures and can also be employed in lower dosages as a laxative to promote elimination and / or to relieve constipation.
Owner:SALIX PHARMA INC
Chitosan composition for prevention and treatment of chemical liver injury and preparation method thereof
ActiveCN104013733AReplenishing Essence and BloodEssence and blood nourishOrganic active ingredientsDigestive systemLiver and kidneyGanoderma lucidum
The invention discloses a chitosan composition for prevention and treatment of chemical liver injury and a preparation method thereof. Main materials of chitosan, the root of kudzu vine, Poria, Ganoderma lucidum, medlar and gardenia are subjected to the medicinal material dry paste powder preparation, of chitosan preparation, mixing granulation and capsule filling and packing, so as to obtain the composition. The chitosan in the formula of the invention can promote the secretion of liver oxidase, activate and regenerate liver cells; the root of kudzu vine, Poria, Ganoderma lucidum, medlar and gardenia are Chinese herbal medicines used as both medicine and food, and have the effects of invigorating liver and kidney, clearing heat, removing dampness, promoting blood circulation and removing blood stasis; and the composition for prevention and treatment of chemical liver injury prepared from the above materials can achieve the effects of addressing both the symptoms and root cause and enhancing curative effect for chemical liver injury. The preparation method is simple, low in industrialized production cost, and easy for implementation, and has optimistic market outlook.
Owner:高益槐 +1
Benzoic acid, benzoic acid derivatives and heteroaryl carboxylic acid conjugates of hydromorphone, prodrugs, methods of making and use thereof
ActiveUS20130150395A1Lower potentialReduced drug abuse potentialBiocideNervous disorderBenzoic acidEpoxy
The presently described technology provides compositions comprising aryl carboxylic acids chemically conjugated to hydromorphone (4,5-α-epoxy-3-hydroxy-17-methyl morphinan-6-one) to form novel prodrugs / compositions of hydromorphone. The hydromorphone prodrugs of the present technology have decreased side effects and decreased potential for abuse compared to unconjugated hydromorphone. The present technology also provides methods of treating patients, pharmaceutical kits and methods of synthesizing conjugates of the present technology.
Owner:KEMPHARM INC
Red tangerine peel medicine for treating sputum cough and its preparation method
A Chinese medicine in the form of effervescent tablet for treating cough and resolving sputum is prepared from 8 Chinese-medicinal materials including pummelo peel, bitter almond, liquorice root, etc and 3 medicinal additives: citric acid, dicarbonate and polyethanediol.
Owner:JIANGSU KANION PHARMA CO LTD
Compound ginseng-astragalus immunopotentiator
InactiveCN101019885AGood effectNo side effectsOrganic active ingredientsImmunological disordersIrritationT lymphocyte
The compound ginseng-astragalus immunopotentiator for enhancing immunological function of animal is injection, each 1000 ml of which contains ginsenoside 0.75 g and astragalus polysaccharide 3.0 g except water for injection. The compound ginseng-astragalus immunopotentiator may be used alone to enhance the cellular immunity and humoral immunity or as immunological adjuvant to result in excellent immunopotentiating effect. Pharmacological test shows that the immunopotentiator can raise the expression of chicken's peripheral blood T lymphocyte IL-2mRNA and IFN-gamma-mRNA. Safety test shows that the immunopotentiator has no irritation and no toxicity.
Owner:NANJING AGRICULTURAL UNIVERSITY
Insecticidal composition
InactiveCN103947646ASynergisticGood quick effectBiocideAnimal repellantsChlorfenapyrActive component
The invention relates to an insecticidal composition which comprises two active components including cyhalodiamide and chlorfenapyr. The insecticidal composition has obvious synergism for controlling rice-stem borer, and not only is capable of improving the controlling effect, but also is capable of reducing the dosage, and delaying the drug resistance, wherein the lasting period is prolonged.
Owner:BEIJING YOLOO BIO TECH CORP
External medicine composition for treating traumatic injuries and arthralgia syndrome and preparation method of external medicine composition
ActiveCN103432518AGood treatment effectGood curative effectAntipyreticAerosol deliveryJoint arthralgiaCleansing Agents
The invention discloses external medicine composition for treating traumatic injuries and arthralgia syndrome, which comprises the following components in parts by weight: 100-350 parts of pseudo-ginseng, 68-200 parts of aconite root, 2-15 parts of curcuma zedoary, 2-15 parts of radix angelicae, 1-10 parts of red paeony root, 1-10 parts of rhizoma corydalis and 1-10 parts of safflower. The invention further discloses the preparation methods of ointment, gels, cleaning agent and cream of the external medicine composition. The external medicine composition for treating traumatic injuries and arthralgia syndrome, which is disclosed by the invention, is reasonable in formula, simple in technology, easy to operate, applicable to the treatment of traumatic injuries and arthralgia syndrome and suitable for industrial production, and has the excellent efficacy of promoting blood circulation to remove blood stasis, relieving swelling and pain, and removing blood stasis for promoting tissue regeneration.
Owner:JIANGSU QINGJIANG PHARMA
Medicament for treating colonitis
ActiveCN101590216ADetermine the nature of the lesionQuality assuranceDigestive systemAluminium/calcium/magnesium active ingredientsSal ammoniacChronic colitis
The invention discloses a medicament for treating colonitis, which is prepared from the following bulk drugs by weight: 200 portions of calcite (calcined), 40 portions of halitum (calcined), 400 portions of clematis aethusifolia (calcined), 400 portions of rhododendron micranthum (calcined), 5 portions of nutmeg, 5 portions of coriander fruit, 5 portions of rhizoma zingiberis, 5 portions of long pepper, 5 portions of dwarf lilyturf root, 1 portion of pepper, 5 portions of dried white turnip, 5 portions of sal ammoniac, 5 portions of purple sal ammoniac, 5 portions of elecampane, and 10 portions of potassium nitrate. The formulation is a pure Chinese medicament preparation, has small toxic side effect, is suitable for the characteristic of chronic colonitis recurrent attacks, can be taken for a long time, and has remarkable curative effect; besides the formulation adopts a dosage form of enteric-coated granules to avoid the first pass effect in stomach absorption and act on affected parts directly, and each medicinal material in the formulation adopts the principle of relieving diarrhea with astringents and treating both principal and secondary aspects of a disease to achieve the aim of treatment, thus the medicament for treating colonitis is surely a classic anaesthetic worthy of development in clinical application.
Owner:INNER MONGOLIA TIANQI HAN&MONGOLIA PHARMA CO
Compositions of efavirenz
ActiveUS20140220140A1Improve distributionEffective treatmentOrganic active ingredientsPowder deliveryHydrophilic polymersRetroviral infection
The present inventions relates to a solid composition and an aqueous dispersion comprising nanoparticles of the anti-retroviral drug efavirenz. The solid composition and aqueous dispersion additionally comprise a mixture of a hydrophilic polymer and a surfactant. The surfactant is selected from vitamin-E-polyethylene glycol-succinate (Vit-E-PEG-succinate), a polyoxyethylene sorbitan fatty acid ester, N-alkyldimethylbenzylammonium chloride, sodium deoxycholate, dioctyl sodium sulfosuccinate, polyethyleneglycol-12-hydroxystearate, polyvinyl alcohol (PVA), and a block copolymer of polyoxyethylene and polyoxypropylene, or a combination thereof. The hydrophilic polymer is suitably selected from polyvinyl alcohol (PVA), a polyvinyl alcohol-polyethylene glycol graft copolymer, a block copolymer of polyoxyethylene and polyoxypropylene, polyethylene glycol, hydroxypropyl methyl cellulose (HPMC), and polyvinylpyrrolidone, or a combination thereof. The present invention also relates to processes for preparing both the solid composition and the aqueous dispersion, as well as to their use in therapy for the treatment and / or prevention of retroviral infections such as human immunodeficiency virus (HIV).
Owner:UNIV OF LIVERPOOL
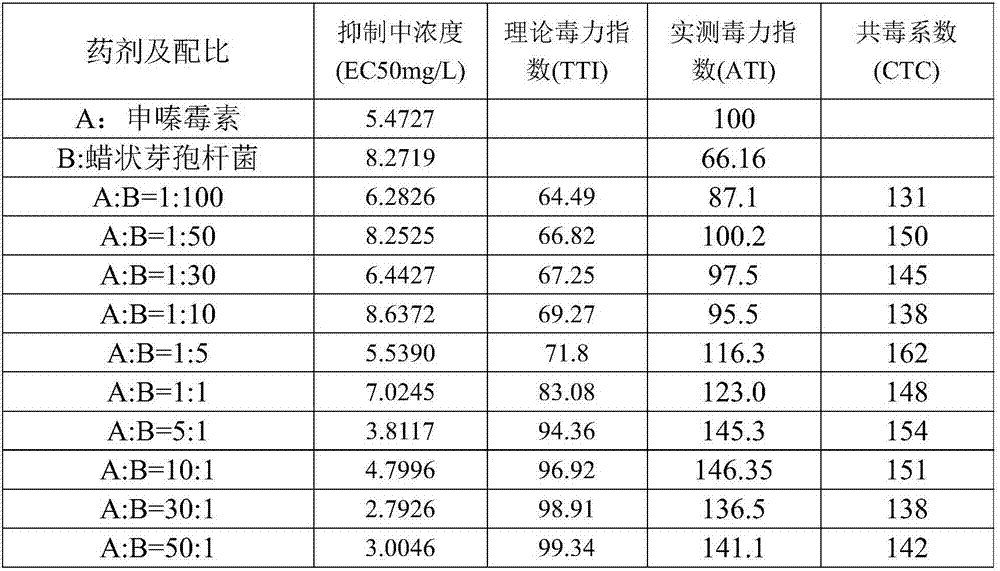
Phenazine-1-carboxylic acid-based bactericidal composition and a bactericide containing the same
InactiveCN107306999AGood control effectWide range of sterilizationBiocideFungicidesActive componentPhenazine
The present invention relates to a phenazine-1-carboxylic acid-based bactericidal composition and a bactericide containing the same, wherein the active component of the bactericidal composition is phenazine-1-carboxylic acid and Bacillus cereus, a weight ratio of the phenazine-1-carboxylic acid to the Bacillus cereus is 1:100-100:1, the Bacillus cereus content in per g of the bactericidal composition is 10<6>-10<11> / g, and the bactericide contains 1-90% by weight of the bactericidal composition. Compared to the bactericidal composition in the prior art, the bactericidal composition of the present invention has advantages of significant synergistic effect, safety, environmental protection, toxicity reducing, pollution reducing, pesticide application cost reducing, economy, practicality, effective delaying of the drug resistance of bacteria, agent consumption reducing, and good application prospect.
Owner:SHANGHAI NONGLE BIOLOGICAL PROD
Unit dosage forms of temozolomide
InactiveUS20080319039A1Reduced pill burdenPatient compliance is goodOrganic active ingredientsBiocideDiseaseTemozolomida
This invention relates to unit dosage forms of temozolomide. These unit dosage forms are particularly well-suited for decreasing the pill burden and increasing patient compliance. The invention also relates to methods of treating proliferative disorders in a patient with these unit dosage forms. The invention additionally relates to kits comprising these unit dosage forms.
Owner:MERCK SHARP & DOHME CORP
Compound stemona cough stopping medicine and its preparation method
ActiveCN1559527AAdvanced dosage formDosage stableUnknown materialsPill deliveryLiquoricesGLYCYRRHIZA EXTRACT
A Chinese medicine in the form of effervescent tablet for treating cough is prepared from 11 Chinese-medicinal materials including bitter almond, mulberry bark, tangerine peel, liquorice root, etc and 3 medicinal additives: citric acid, dicarbonate and polyethanediol 6000.
Owner:JIANGSU KANION PHARMA CO LTD
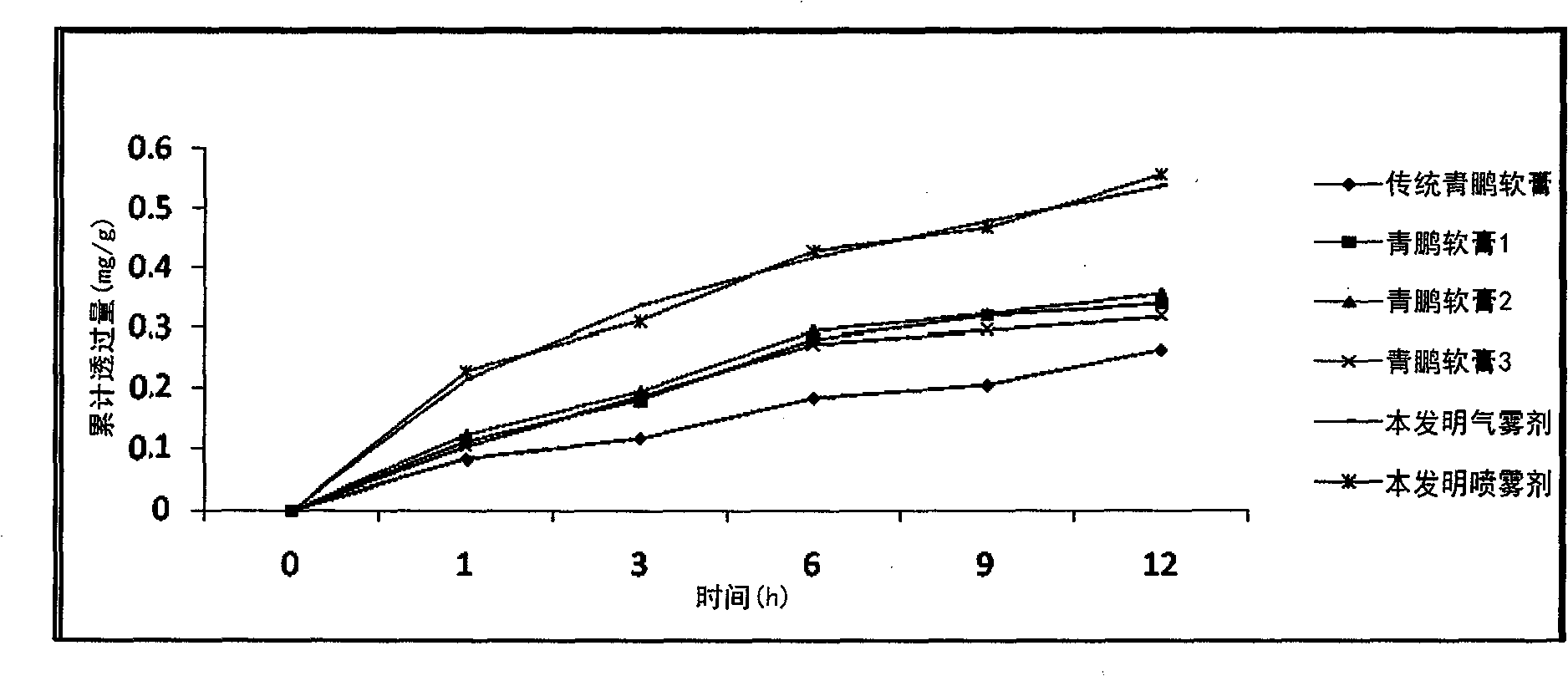
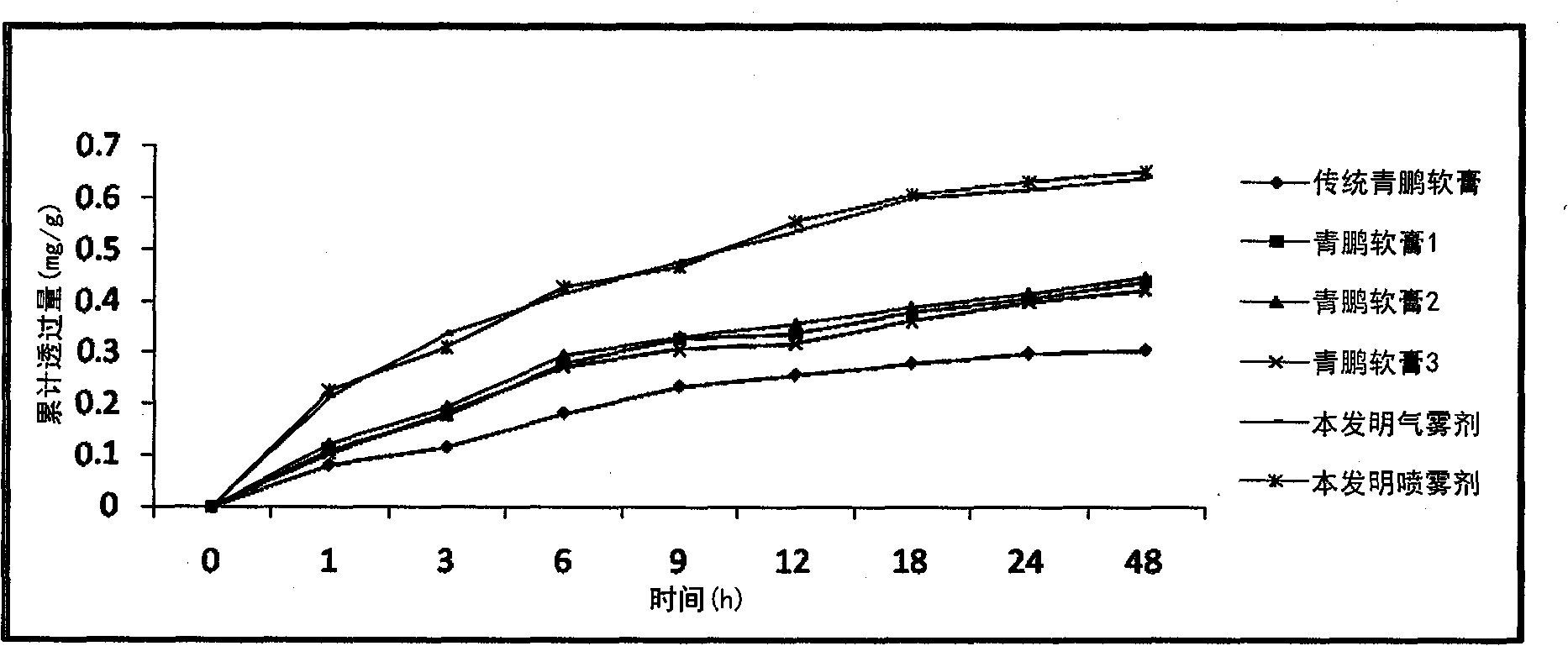
Qingpeng spray preparation for relieving pain and swelling and preparation method thereof
ActiveCN102100757AReduce pollutionReduce spoilageAntipyreticAerosol deliveryCurative effectBULK ACTIVE INGREDIENT
The invention provides a spray preparation for relieving pain and swelling, which comprises active ingredients and a substrate, wherein the active ingredients are prepared from the following raw material medicaments in part by weight: 80 to 120 parts of oxytropis, 30 to 70 parts of sub-rhubarb, 50 to 100 parts of tiebangchui aconite tuber, 80 to 120 parts of core removal medicine terminalia fruit, 80 to 120 parts of terminalia bellerica, 80 to 120 parts of emblic leafflower fruit, 20 to 50 parts of benzoin, 130 to 170 parts of Chinese tinospora stem and 10 to 40 parts of musk; and the substrate comprises the following auxiliary materials in part by weight: 100 to 800 parts of excipient and 50 to 500 parts of emulsifier. The spray preparation has the characteristics of advanced formulations, simplicity and convenience for process, quick response and the like. On the basis of ensuring a curative effect, the quality of products is improved, beneficiaries are enlarged, and the quality of the products is safer, more stable and controllable.
Owner:TIBET QIZHENG TIBETAN MEDICINE
Pesticide composition
The invention belongs to the technical field of pesticides, and relates to a pesticide composition. The pesticide composition is characterized in that active components comprise chlorobenzene ether amide and metalaxyl-M. The composition can be prepared into a suspoemulsion, so that the synergistic effect is obvious, generation of drug resistance is delayed, the lasting period is prolonged, and the pesticide composition is environment-friendly.
Owner:燕化永乐(乐亭)生物科技有限公司
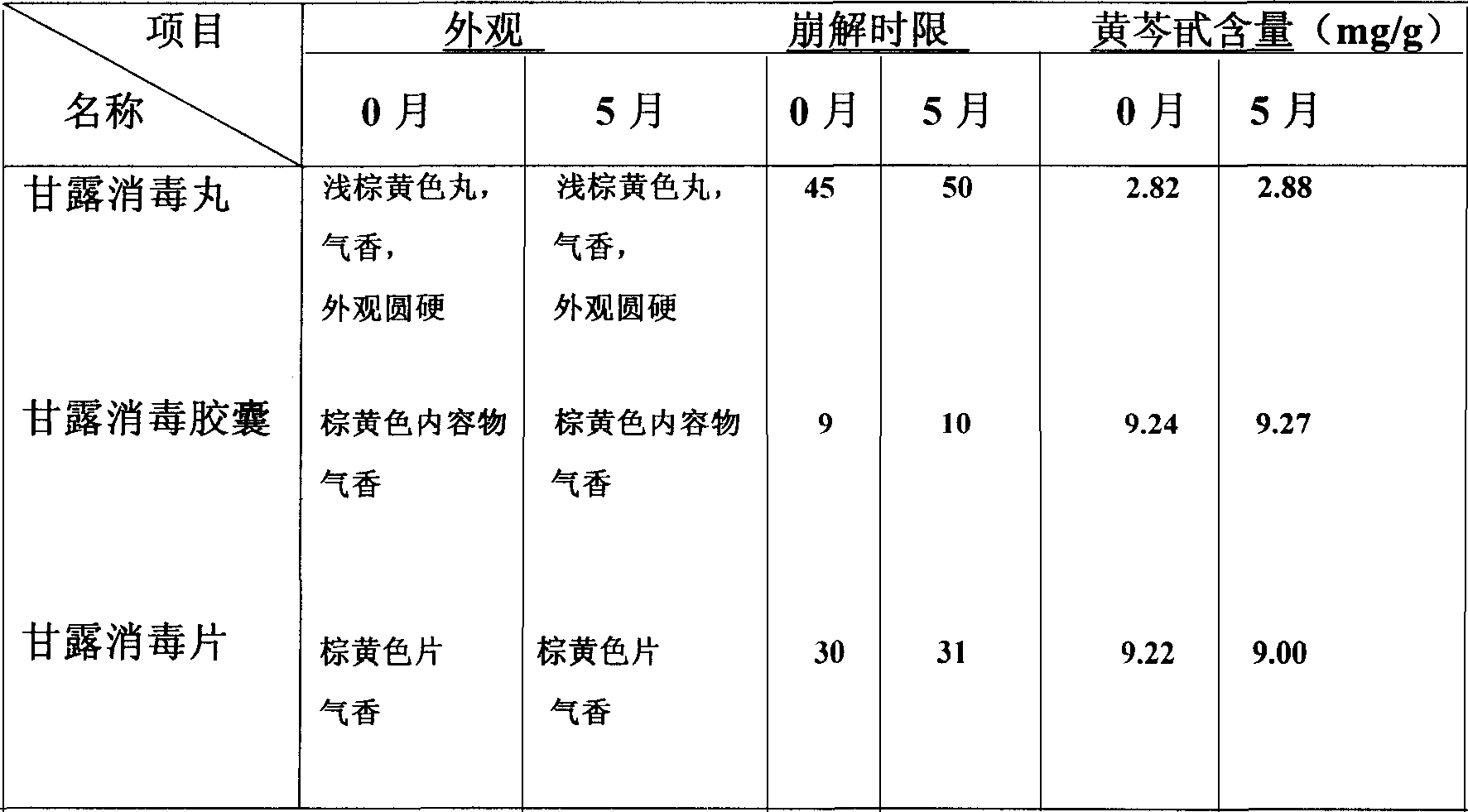
Heat clearing Chinese proprietary capsule(tablet) of 'Ganlu Xiaodu' for treating cold and its preparation process
The invention discloses a heat clearing Chinese proprietary capsule (tablet) of 'Ganlu Xiaodu' for treating cold and its preparation process, which comprises eleven kinds of Chinese medicinal herbs including baikal skullcap root, talcum powder, grassleaved sweetflag rhizome and belamcanda rhizome. The preparing process comprises making extract by prescription contents, mixing 100 parts of extract with 2-5 parts of silicon dioxide to prepare capsule, or mixing 100 parts of extract with 0.2-0.5 part of magnesium stearate to obtain tablets, and the content of the active ingredient of baicalin in the extract in no less than 9.0%.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
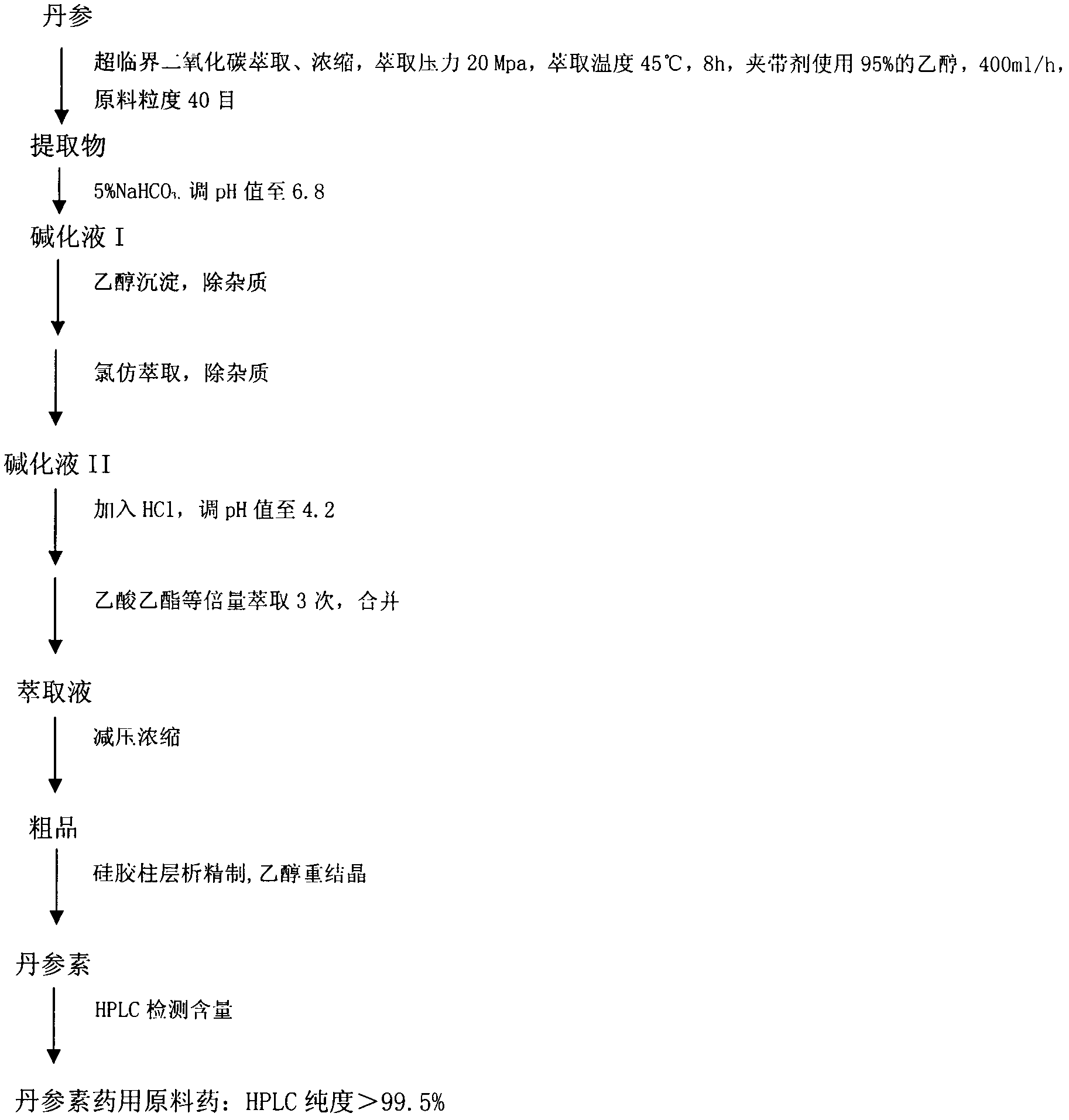
Compound salvianic acid injection for treating cerebral vascular thrombosis diseases and preparation process thereof
InactiveCN102379864AImprove clinical efficacyQuality improvementPowder deliveryAntipyreticDiseaseCoronary heart disease
The invention aims to provide a compound salvianic acid injection which uses salvianic acid and paeonol as main components with high efficiency, advanced dosage form and stable preparation, as well as a preparation process thereof, and belongs to the field of biological pharmacy. The method comprises the following steps: extracting salvianic acid from salvia, and extracting paeonol from peony bark and enamelling with 2-hydroxypropyl-beta-cyclodextrin to prepare paeonol- hydroxypropyl-beta-cyclodextrin clathrate compound; weighting and mixing the salvianic acid, the paeonol clathrate compound and an excipient according to marked weights in the prescription, dissolving with injection water, filtering, and regulating the pH of the filtrate to be between 6.8 and 7.2; sterilizing and filtering, and diluting with aseptic injection water to the total designing solution; and packaging in a brown glass tube vial, and lyophilizing. The injection has the effects of activating blood circulation to remove stasis, and dredging collaterals and relieving pain, and is suitable for patients with cerebral vascular thrombosis diseases, such as coronary heart disease, angina pectoris, cerebral thrombosis, cerebral infarction and the like. The injection for adults is used for intramuscular injection or intravenous drip, and is dosed 1 to 5 vials one time, 1 to 2 times per day or as professionally prescribed.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Compositions of lopinavir
ActiveUS20140212501A1High drug loadingAdvanced dosage formPowder deliveryOrganic active ingredientsHydrophilic polymersRetroviral infection
The present invention relates to a solid composition and an aqueous dispersion comprising nanoparticles of the anti-retroviral drug lopinavir. The solid composition and aqueous dispersion additionally comprise a mixture of a hydrophilic polymer and a surfactant. The surfactant is selected from vitamin-E-polyethylene glycol-succinate (Vit-E-PEG-succinate), a polyoxyethylene sorbitan fatty acid ester, N-alkyldimethylbenzylammonium chloride, sodium deoxycholate, dioctyl sodium sulfosuccinate, polyethyleneglycol-12-hydroxystearate, polyvinyl alcohol (PVA), and a block copolymer of polyoxyethylene and polyoxypropylene, or a combination thereof. The hydrophilic polymer is suitably selected from polyvinyl alcohol (PVA), a polyvinyl alcohol-polyethylene glycol graft copolymer, a block copolymer of polyoxyethylene and polyoxypropylene, polyethylene glycol, hydroxypropyl methyl cellulose (HPMC), and polyvinylpyrrolidone, or a combination thereof. The present invention also relates to processes for preparing both the solid composition and the aqueous dispersion, as well as to their use in therapy for the treatment and / or prevention of retroviral infections such as human immunodeficiency virus (HIV).
Owner:UNIV OF LIVERPOOL
Single drive type roller press
InactiveCN103098804AFacilitated DiffusionReduce the frequency of sprayingBiocideAnimal repellantsEngineeringDriving mode
The invention discloses a single drive type roller press, which includes a rack. The rack is fixedly provided with a fixed roll and is slidably provided a movable roll, wherein the position of the fixed roll is fixed, and the movable roll and the rack can move relatively to adjust the distance between two rolls. A feeding device is arranged above the fixed roll and the movable roll. The rack is also provided with a thrust system, which is connected to the movable roll. A power system connected to the movable roll is mounted above the rack. The roller press provided in the invention adopts a single drive mode to provide rotation power to the two rolls, its installed power is lower than that of a roller press employing a dual-drive structure. With a simple and reliable structure, the single drive type roller press disclosed in the invention is convenient, economical and practical, and has low installation and maintenance costs.
Owner:BEIJING YOLOO BIO TECH CORP
Soft capsule of dahurian rhododendron leaf and preparation method
InactiveCN1519012AImprove bioavailabilityGood cough suppressantUnknown materialsCapsule deliveryMedicineSteam distillation
A dahurian rhododendron softgel for treating cough and asthma and dispelling phlegm is prepared from dahurian rhododendron through distilling to extract volatile oil, extracting from dregs in alcohol, recovering alcohol, mixing, concentrating, adding auxiliaries, superfine pulverizing and die pressing.
Owner:哈药集团中药二厂
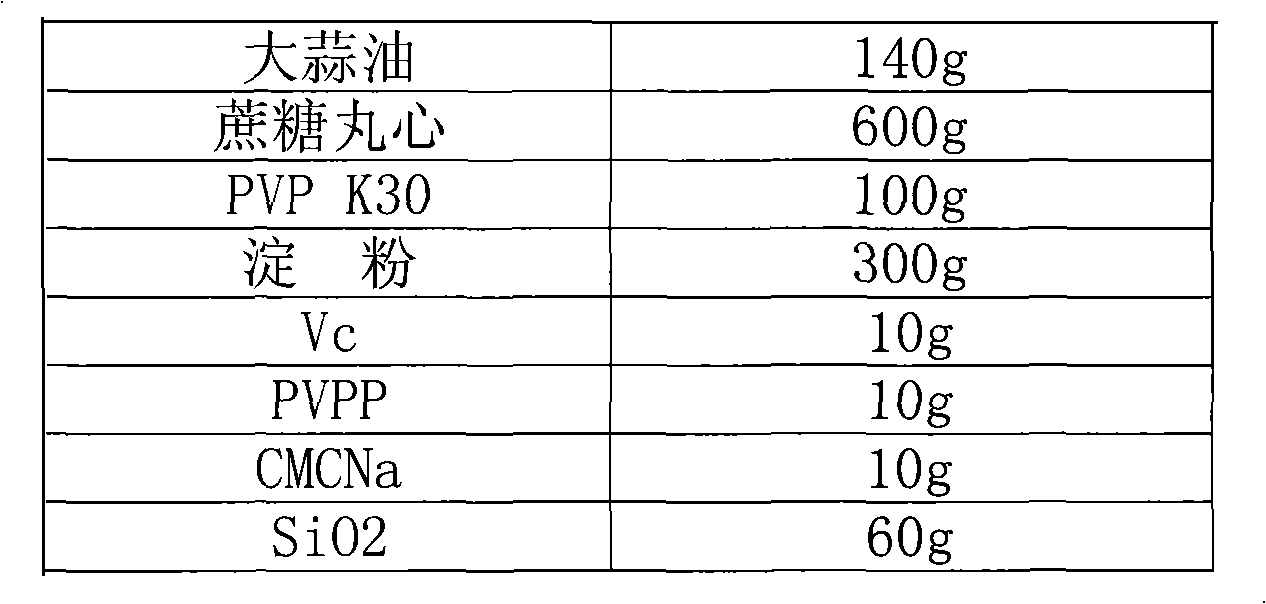
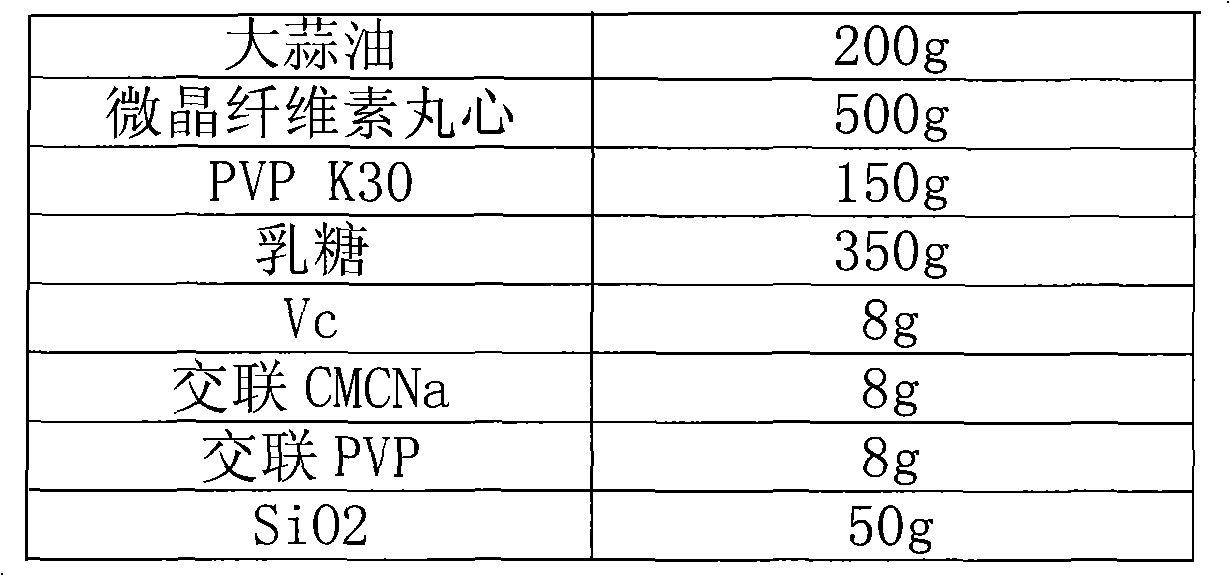
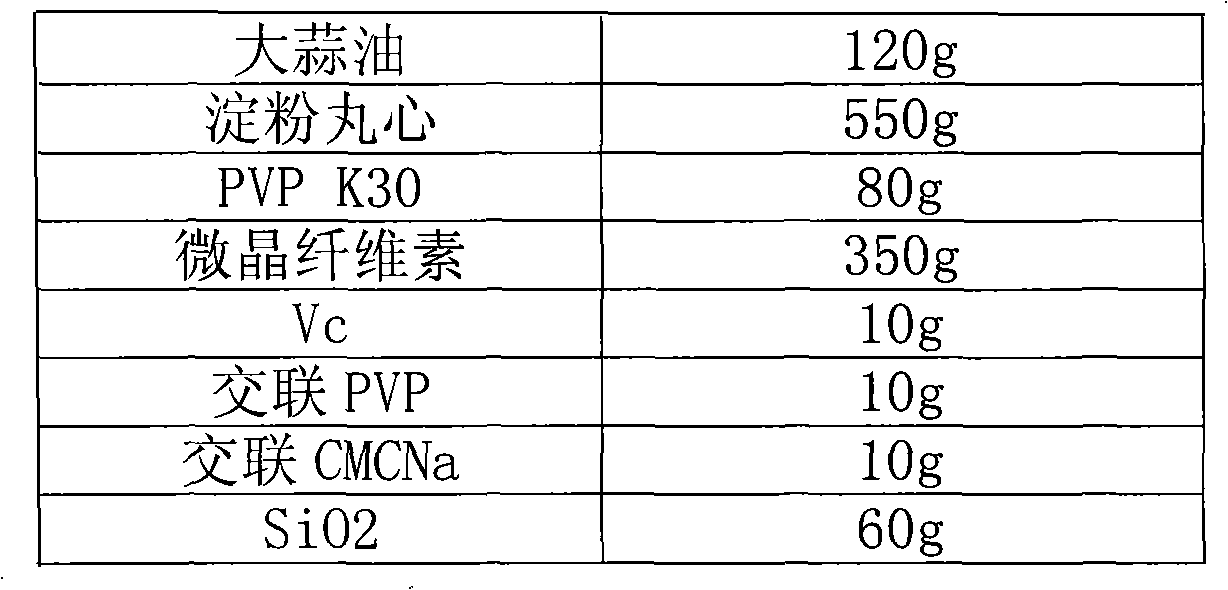
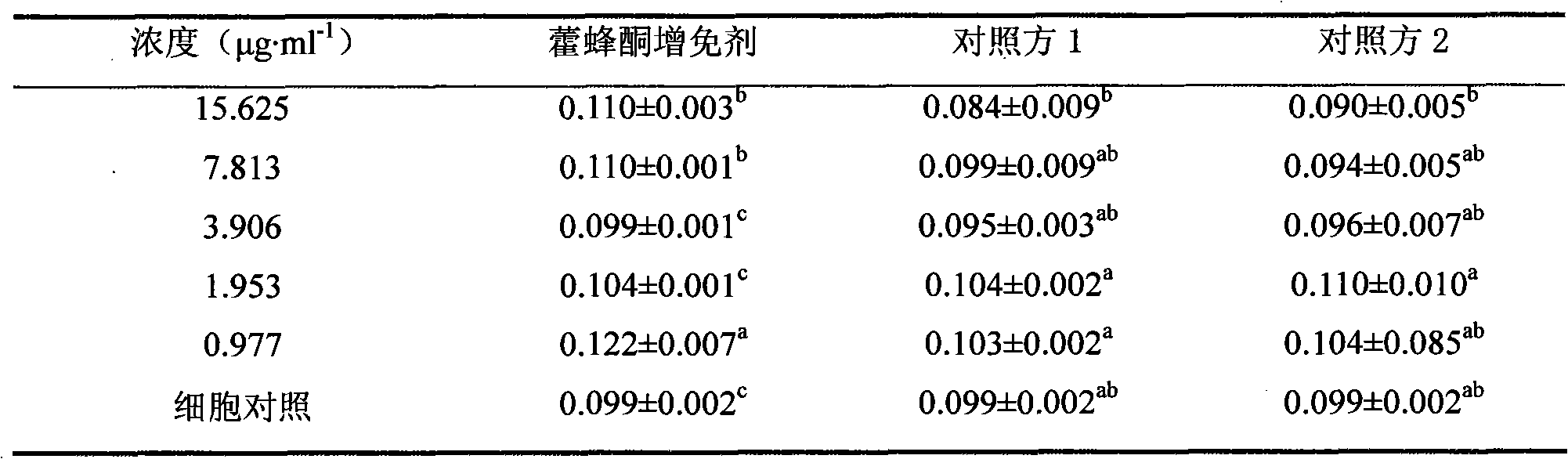
Garlic oil enteric pellet capsule and preparation method thereof
ActiveCN101804145AGood water solubilityPromote absorptionAntibacterial agentsMetabolism disorderAdditive ingredientBULK ACTIVE INGREDIENT
The invention relates to the field of antibacterial agent, in particular to a garlic oil enteric pellet capsule and a preparation method thereof. Wherein the garlic oil pellet capsule is composed of a coated pellets and a coating layer, the coated pellets comprises pharmaceutically active ingredients of garlic oil and coated pellet minor ingredient; wherein the coated pellet minor ingredient comprises pellet core, bonding agent, absorbing agent, disintegrating agent, antioxygen agent and antiplastering aid while the coating layer comprises an enteric coating and an isolated layer; the coated pellet comprises the following components according to weight percentage: 5-40% of garlic oil, 10-60% of pellet core, 2-10% of binding agent, 20-50% of absorbing agent, 0.5-5% of antioxygen agent, 0.5-10% of disintegrating agent and 2-20% of antiplastering aid.Compared with the existing allicin dosage form, the garlic oil enteric pellet capsule of the invention enjoys more advanced dosage form, thus ensuring effectiveness and stability of the capsule and high bioavailability; in addition, with the enteric pellet capsule of the invention, harm done to gastric mucosa as the capsule is dissolved inside the stomach is avoided and the allicin is not damaged by gastric acid so that drug effect of the capsule is not affected.
Owner:HAINAN PULIN PHARMA
Chinese medicinal herba epimedii and propolis flavone immunopotentiator
ActiveCN101869590AGood effectNo pollution in the processAnthropod material medical ingredientsImmunological disordersPropolisCellular immunity
The invention relates to a Chinese medicinal herb epimedii and propolis flavone immunopotentiator, which is called the herb epimedii and propolis flavone immunopotentiator for short and belongs to the field of immunologic adjuvant for livestock and poultry. Each 1,000 ml of liquid medicine is prepared from 15 grams of herb epimedii and 5 grams of propolis flavone by the following steps of: adding water, decocting the herb epimedii twice, merging percolate and concentrating the percolate to prepare 870 ml of herb epimedii extract; dissolving propolis, which is processed with rice wine, with ethanol, filtering and concentrating the dissolved propolis to prepare 130 ml of propolis; and mixing the 130 ml of propolis and the herb epimedii extract to prepare the product. Immunity tests prove that the herb epimedii and propolis flavone immunopotentiator can remarkably stimulate the multiplication of lymphocytes of chicken in vitro, remarkably improve the antibody tilter of serum when used together with Newcastle vaccine for chicken, promote the multiplication of the lymphocytes, enhance the cellular immunity and humoral immunity of the chicken and improve the immune response of vaccines.
Owner:NANJING AGRICULTURAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com