Compound salvianic acid injection for treating cerebral vascular thrombosis diseases and preparation process thereof
The technology of a plain injection and compound danshen is applied in the field of compound danshensu injection, which can solve the problems such as difficulty in dissolving paeonol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
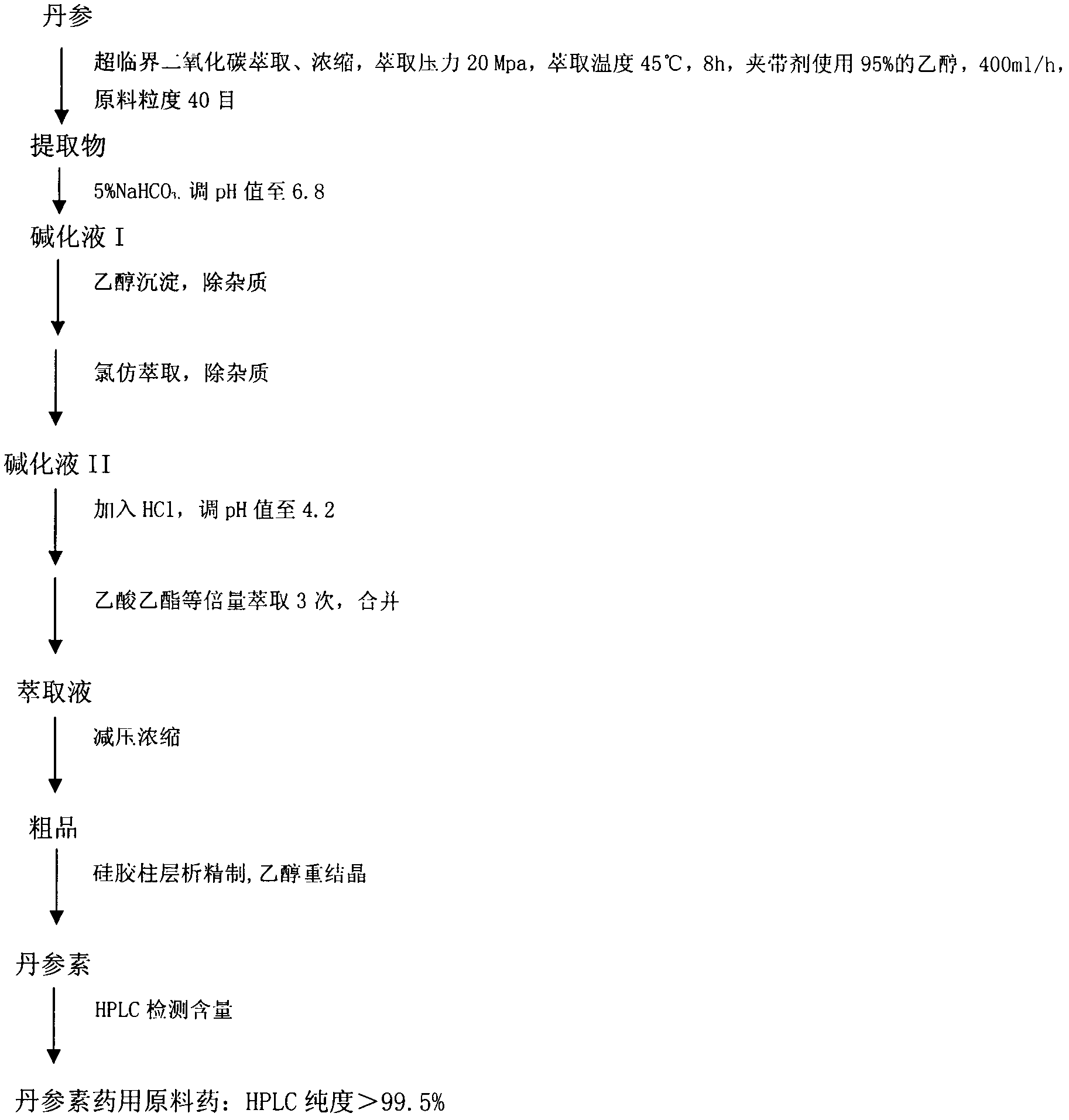
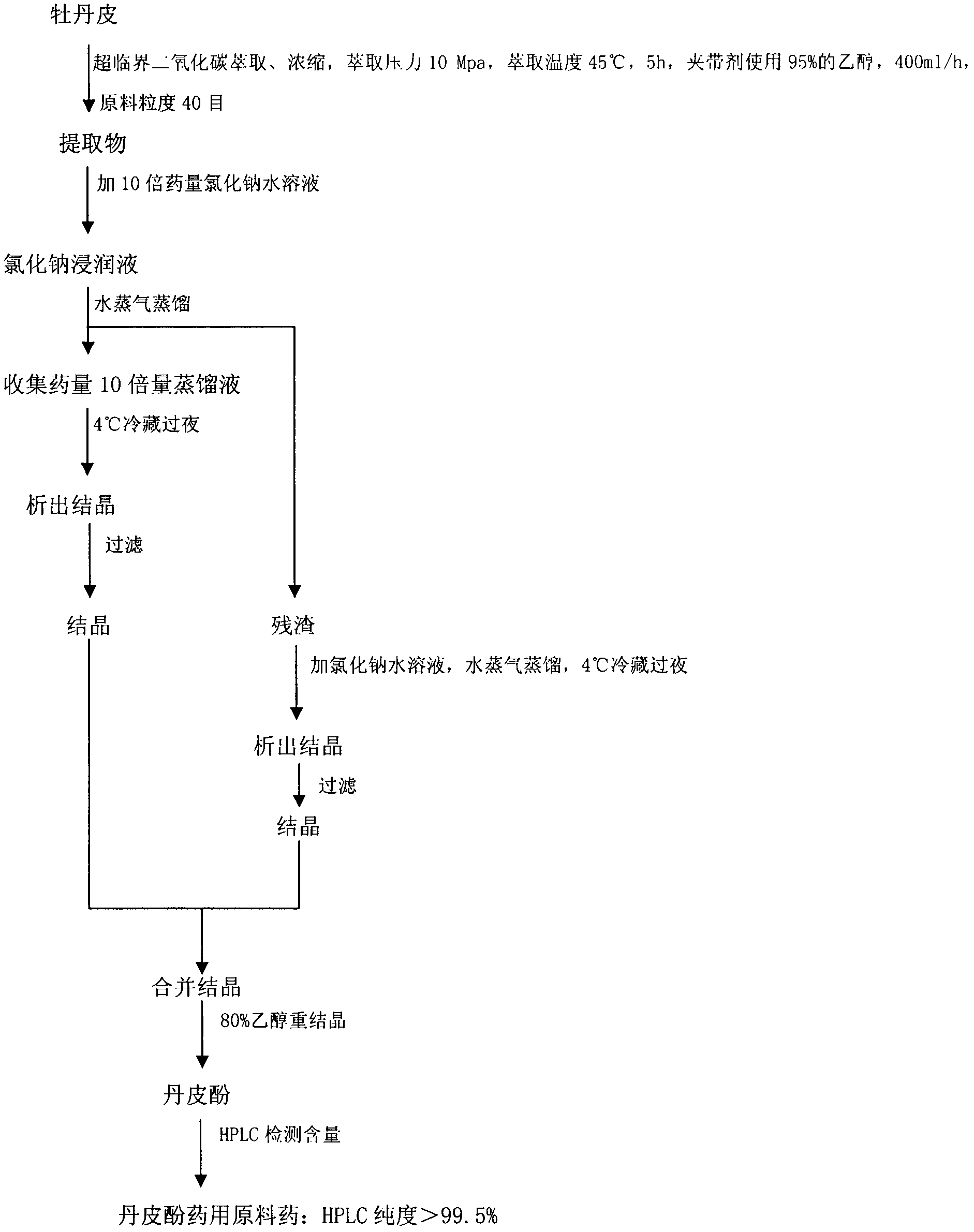
[0018] 4. The preparation method of compound danshensu injection
[0019] (1) In parts by weight, take 1 part of paeonol and dissolve it with 20 parts of absolute ethanol; take another 6 parts of hydroxypropyl-β-cyclodextrin, add water for injection in a water bath at 50°C to make a saturated solution; Slowly add paeonol absolute ethanol solution dropwise to hydroxypropyl-β-cyclodextrin at a rate of 3 drops / min, keep stirring at the temperature of the water bath until the absolute ethanol evaporates, sonicate for 1 hour, and refrigerate at 4°C Overnight, filter, and dry the filter residue at 40°C to obtain paeonol-hydroxypropyl-β-cyclodextrin inclusion compound; measure the encapsulation efficiency and the content of paeonol in the inclusion compound.
[0020] (2) Weigh danshensu, paeonol inclusion compound and excipients according to the weight indicated on the prescription, mix them, dissolve them with water for injection, filter, adjust the pH of the filtrate to 6.8-7.2, st...
example 1
[0026] [Prescription] Danshensu 100g Paeonol 1g Mannitol 60g
[0027] [Preparation process] (1) Take 1g of paeonol and dissolve it in 20mL of absolute ethanol; take another 6g of hydroxypropyl-β-cyclodextrin and add water for injection in a water bath at 50°C to make a saturated solution; Slowly add absolute ethanol solution to hydroxypropyl-β-cyclodextrin at a rate of 3 drops / min, keep stirring at the temperature of the water bath until the absolute ethanol evaporates, sonicate for 1 hour, refrigerate overnight at 4°C, and filter , and the filter residue was dried at 40°C to obtain paeonol-hydroxypropyl-β-cyclodextrin inclusion compound.
[0028] (2) Weigh danshensu, paeonol inclusion compound and excipients according to the weight indicated on the prescription, mix them, dissolve them with water for injection, filter, adjust the pH of the filtrate to 6.8-7.2, sterilize, filter, and use aseptic injection Dilute it with water to the designed total volume, divide it into brown...
example 2
[0030] [Prescription] Danshensu 100g Paeonol 5g Mannitol 70g
[0031] [Preparation process] (1) Take 5g of paeonol and dissolve it in 100mL of absolute ethanol; take another 30g of hydroxypropyl-β-cyclodextrin, add water for injection in a water bath at 50°C to make a saturated solution; Slowly add absolute ethanol solution to hydroxypropyl-β-cyclodextrin at a rate of 3 drops / min, keep stirring at the temperature of the water bath until the absolute ethanol evaporates, sonicate for 1 hour, refrigerate overnight at 4°C, and filter , The filter residue was dried at 40°C to obtain paeonol-hydroxypropyl-β-cyclodextrin inclusion compound.
[0032] (2) Weigh danshensu, paeonol inclusion compound and excipients according to the weight indicated on the prescription, mix them, dissolve them with water for injection, filter, adjust the pH of the filtrate to 6.8-7.2, sterilize, filter, and use aseptic injection Dilute it with water to the designed total volume, divide it into brown contro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com