Novel method for preparing Nevirapine
A technology of methyl and compound, applied in the new field of preparation of nevirapine, can solve the problems of high reaction temperature, high production cost, difficult recovery, etc., and achieve the effects of high yield, reduced energy consumption, and less dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
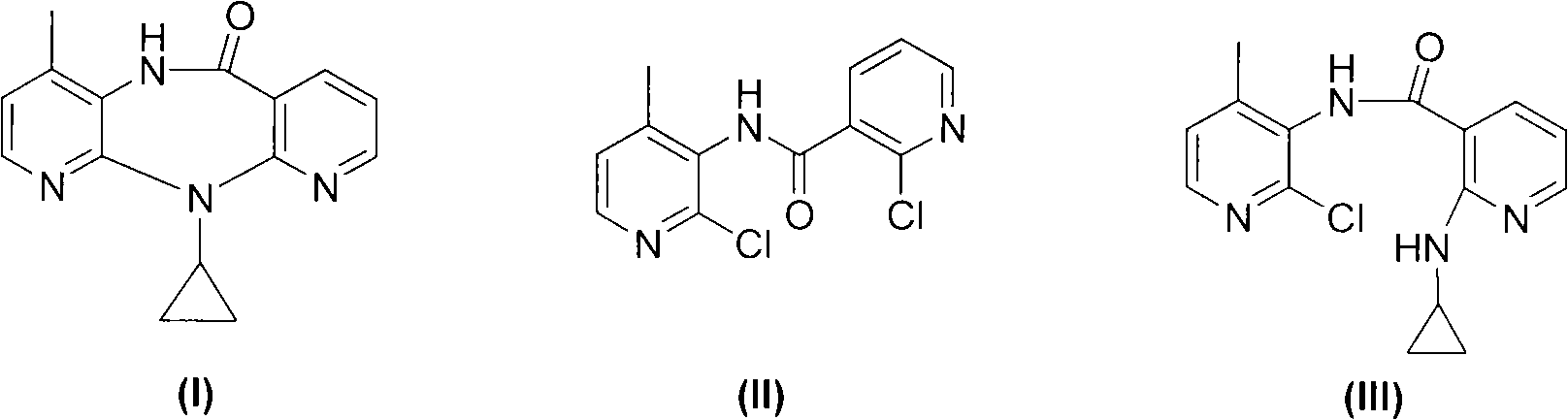
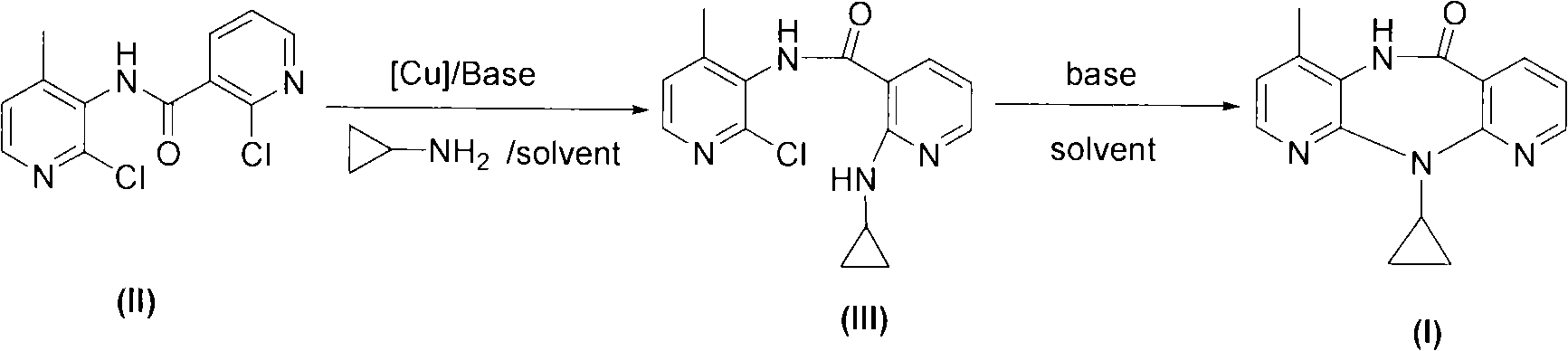
[0017] Add 22.5 g of compound (II), 0.8 g of cuprous chloride, 11 g of sodium carbonate, and 400 ml of toluene into a 1000 ml three-neck reaction flask in sequence, add 18 g of cyclopropylamine under electromagnetic stirring, and stir at 25°C for 15 hours.
[0018] The reaction solution was filtered, the filtrate was dried with anhydrous magnesium sulfate, filtered, the solvent in the filtrate was evaporated to dryness under reduced pressure to obtain a light brown solid, which was compound (III), and the HPLC detection content was 92%, which was directly used in the next step reaction . The obtained compound (III) was dissolved in 100 ml of diethylene glycol dimethyl ether for use.
[0019] Add 11 grams of NaH into a 500 ml dry three-necked flask, then add 100 ml of diethylene glycol dimethyl ether, stir and raise the temperature to 120°C, then add the obtained compound (III) diethylene glycol dimethyl ether solution dropwise, dropwise After the addition, continue to keep st...
Embodiment 2
[0021] Add 22.5 g of compound (II), 1.0 g of cuprous bromide, 11 g of sodium carbonate, and 400 ml of toluene into a 1000 ml three-necked reaction flask in sequence, add 18 g of cyclopropylamine under electromagnetic stirring, and stir at 25°C for 15 hours.
[0022] The reaction solution was filtered, the filtrate was dried with anhydrous magnesium sulfate, filtered, and the solvent in the filtrate was evaporated to dryness under reduced pressure to obtain a light brown solid, which was compound (III), which was directly used in the next reaction. The obtained compound (III) was dissolved in 100 ml of diethylene glycol dimethyl ether for use.
[0023] Add 11 grams of NaH into a 500 ml dry three-necked flask, then add 100 ml of diethylene glycol dimethyl ether, stir and raise the temperature to 120°C, then add the obtained compound (III) diethylene glycol dimethyl ether solution dropwise, dropwise After the addition, continue to keep stirring for 1 hour. Lower the temperature ...
Embodiment 3
[0025] Add 22.5 g of compound (II), 1.2 g of cuprous iodide, 11 g of sodium carbonate, and 400 ml of toluene into a 1000 ml three-necked reaction flask in sequence, add 18 g of cyclopropylamine under electromagnetic stirring, and stir at 25°C for 15 hours.
[0026] The reaction solution was filtered, the filtrate was dried with anhydrous magnesium sulfate, filtered, and the solvent in the filtrate was evaporated to dryness under reduced pressure to obtain a light brown solid, which was compound (III), which was directly used in the next reaction. The obtained compound (III) was dissolved in 100 ml of diethylene glycol dimethyl ether for use.
[0027] Add 11 grams of NaH into a 500 ml dry three-necked flask, then add 100 ml of diethylene glycol dimethyl ether, stir and raise the temperature to 120°C, then add the obtained compound (III) diethylene glycol dimethyl ether solution dropwise, dropwise After the addition, continue to keep stirring for 1 hour. Lower the temperature o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com