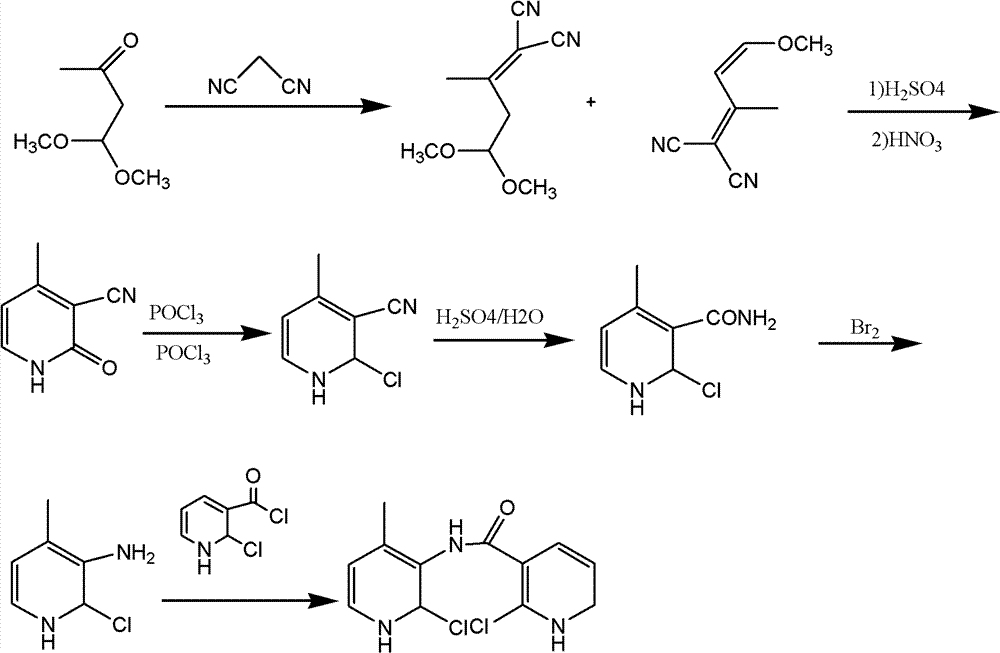
Synthesis method for Nevirapine intermediates
A synthetic method and intermediate technology, applied in the field of intermediate synthesis, can solve the problems of complex reaction processing, environmental damage, etc., and achieve the effects of high yield, reduced pollution, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
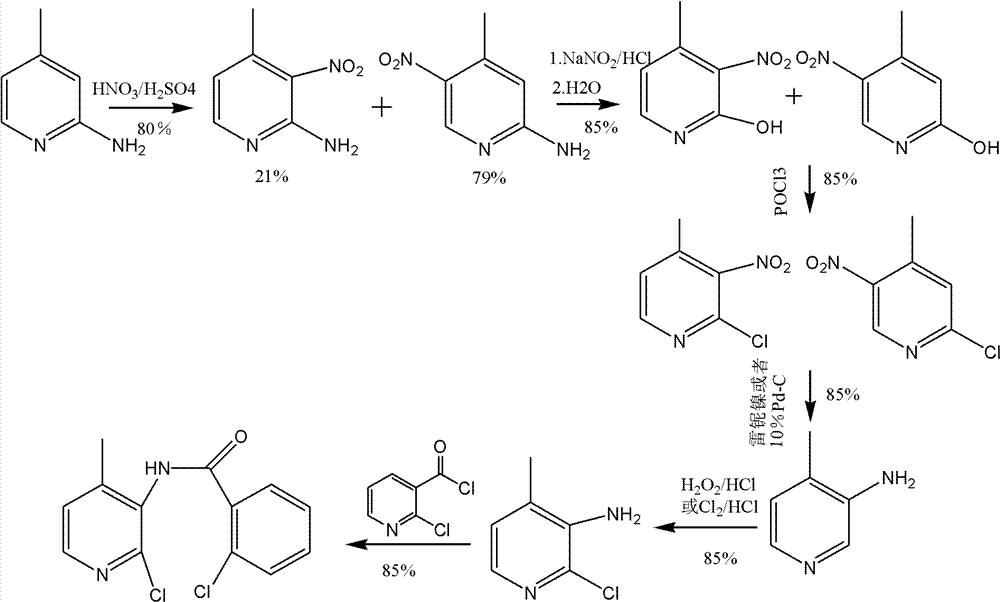
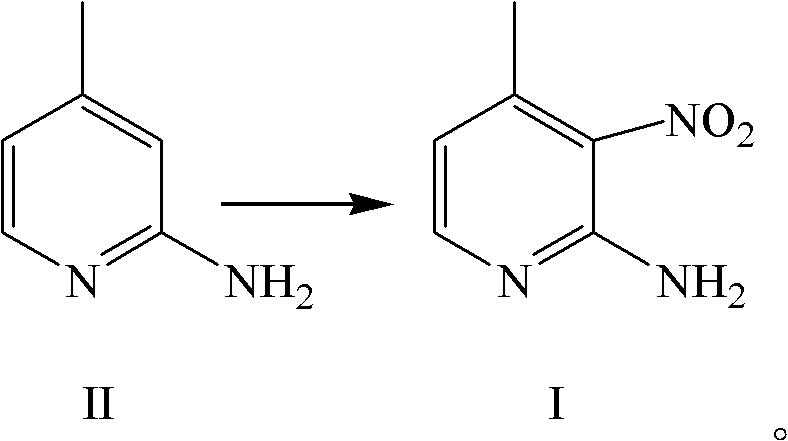
[0042] (1) Synthesis of 2-amino-3-nitro-4-picoline
[0043] In a reaction flask, add 200ml of toluene and 1000g of 95% concentrated sulfuric acid, start stirring, and slowly add 108g of 2-amino-4-picoline and 10g of barium hydroxide in batches at 25°C, and stir until completely dissolved. Then, 330 g of 20% dilute nitric acid was slowly added dropwise, and after the addition was completed, the mixture was incubated at 25° C. for 5 hours. That is the reaction solution.
[0044] In another reaction bottle, add 1000ml of water, start stirring, cool to 10°C, slowly add the above reaction solution into water, control the temperature at 10°C, after the addition is complete, adjust the pH to 8-8.5 with ammonia water, collect the solid, A mixture of 2-amino-3-nitro-4-picoline and 2-amino-4-methyl-5-nitropyridine is obtained. Its molar ratio is: 2-amino-3-nitro-4-picoline:2-amino-4-methyl-5-nitropyridine=100:5.
[0045] The above mixture was recrystallized from absolute ethanol to o...
Embodiment 2
[0055] (1) Synthesis of 2-amino-3-nitro-4-picoline
[0056] In a reaction flask, add 200ml of xylene and 1000g of concentrated sulfuric acid, start stirring, and slowly add 108g of 2-amino-4-picoline and 10g of zinc hydroxide in batches at 35°C, and stir until completely dissolved. Then, 220 g of 30% dilute nitric acid was slowly added dropwise, and after the addition was completed, the mixture was incubated at 35° C. for 5 hours. That is the reaction solution.
[0057] In another reaction bottle, add 1000ml of water, start stirring, cool to 10°C, slowly add the above reaction solution into water, control the temperature at 10°C, after the addition is complete, adjust the pH to 8-8.5 with ammonia water, collect the solid, A mixture of 2-amino-3-nitro-4-picoline and 2-amino-4-methyl-5-nitropyridine is obtained. Its molar ratio is: 2-amino-3-nitro-4-picoline:2-amino-4-methyl-5-nitropyridine=100:4.
[0058] The above mixture was recrystallized with propanol to obtain 146.50 g ...
Embodiment 3
[0068] (1) Synthesis of 2-amino-3-nitro-4-picoline
[0069] In a reaction flask, add 150ml and 1000g of toluene concentrated sulfuric acid, start stirring, and slowly add 108g of 2-amino-4-picoline and 8g of aluminum hydroxide in batches at 45°C, and stir until completely dissolved. Then, 165 g of 40% dilute nitric acid was slowly added dropwise, and after the addition was completed, it was incubated at 45° C. for 5 hours. That is the reaction solution.
[0070] In another reaction bottle, add 1000ml of water, start stirring, cool to 10°C, slowly add the above reaction solution into water, control the temperature at 10°C, after the addition is complete, adjust the pH to 8-8.5 with ammonia water, collect the solid, A mixture of 2-amino-3-nitro-4-picoline and 2-amino-4-methyl-5-nitropyridine is obtained. Its molar ratio is: 2-amino-3-nitro-4-picoline:2-amino-4-methyl-5-nitropyridine=100:3.
[0071] The above mixture was recrystallized from tert-butanol to obtain 148.0 g of pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com