Fluorescent molecular probe for detecting fluoride ions as well as synthesis method and application thereof
A fluorescent molecular probe and fluoride ion technology, applied in the field of chemical analysis and detection, can solve the problems of limiter application, limitation, fluorescence intensity quenching, etc., and achieve the effects of wide detection range, low detection lower limit and high synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of compound 2
[0029] In a 100 mL single-neck round bottom flask, add 2-hydroxy-1-naphthaldehyde (172 mg, 1 mmol) and triethylamine (121 mg. 1.2 mmol) in 15 mL of dichloromethane, and stir at room temperature for 30 minutes. Tert-butyldimethylsilyl chloride (180 mg, 1.2 mmol) was added to the above reaction system, followed by stirring at room temperature for 2 hours. The reaction solution was poured into 100 mL of water to quench the reaction. It was extracted with 30 mL of dichloromethane, and the organic phase was separated and dried with anhydrous sodium sulfate, and the solvent was distilled off to obtain 237 mg of solid product (yield: 83 %), which was compound 2, which was directly carried out to the next step without purification.
Embodiment 2
[0030] Embodiment 2: Preparation of molecular fluorescent probe
[0031] In a 50 mL single-necked flask, compound 2 (29 mg, 0.1 mmol) and malononitrile (6.6 mg, 0.1 mmol) were dissolved in 10 mL of ethanol, 1 drop of piperidine was added, and reacted at room temperature for 30 minutes. The reaction solution was poured into water, extracted with dichloromethane, dried over anhydrous sodium sulfate, and separated by column chromatography (petroleum ether / ethyl acetate at a volume ratio of 50:1 as eluent) to obtain 14 mg of the product (yield: 42%) is the probe compound.
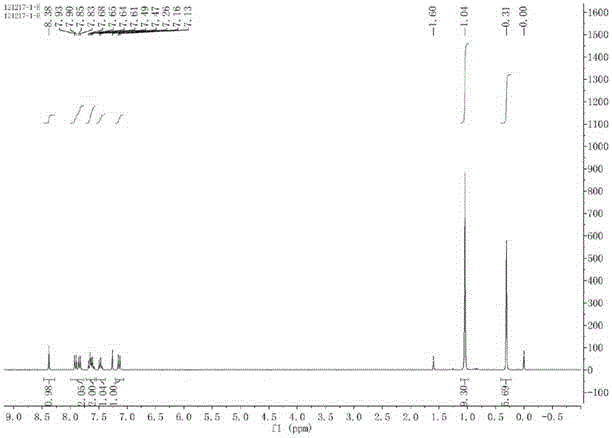
[0032] 1 H NMR (300 MHz, CDCl 3 ) δ 8.38 (s, 1H), 7.91 (d,J = 9.0 Hz, 1H), 7.84 (d, J = 8.1 Hz, 1H), 7.72 – 7.56 (m, 2H), 7.47 (t, J = 7.3 Hz, 1H), 7.14 (d, J = 9.0 Hz, 1H), 1.04 (s, 9H), 0.31 (s, 6H).
Embodiment 3
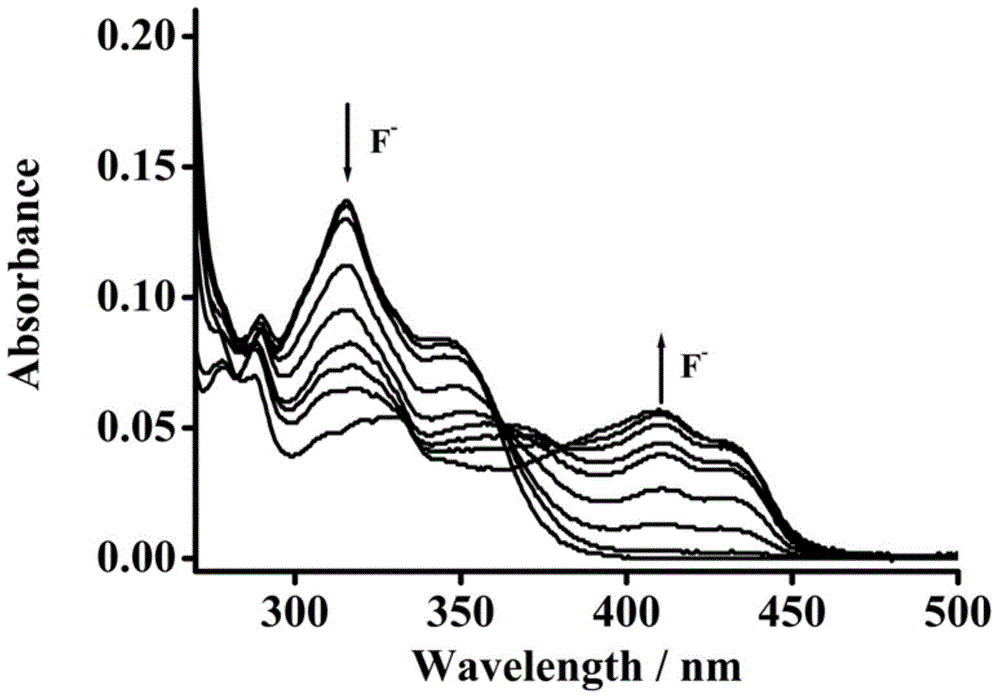
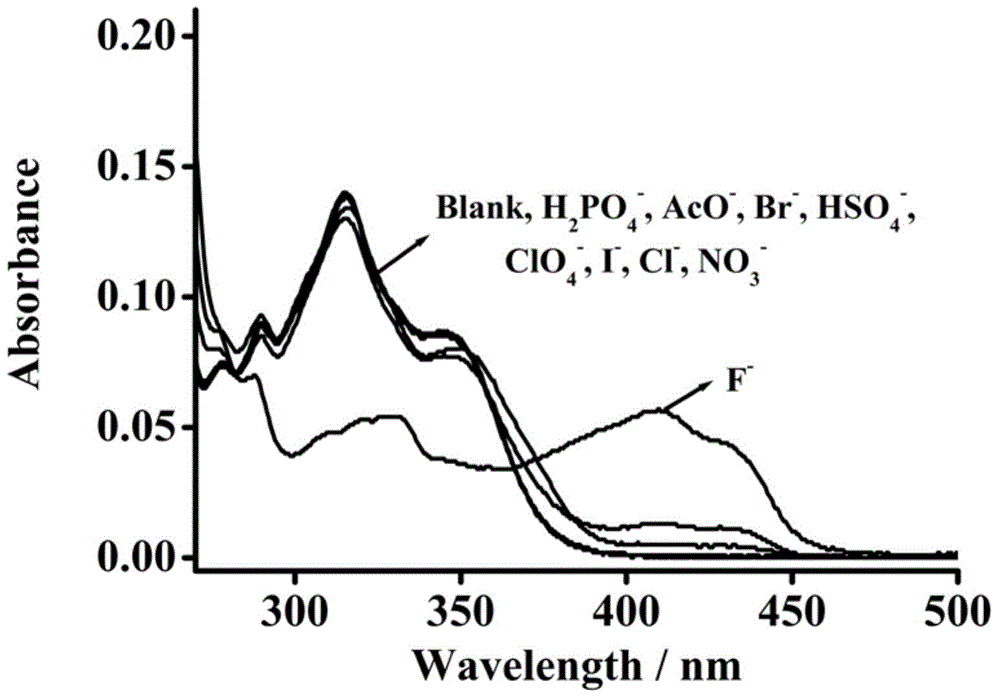
[0033] Example 3: Application of Naked Eye Recognition and Detection of Fluoride Ion Fluorescent Molecular Probes
[0034] The probe was dissolved in acetonitrile solution at a concentration of 25 μM, and different anions (25 μM of fluoride, dihydrogen phosphate, acetate, bromide, hydrogen sulfate, chlorate, iodide, chloride, Nitrate) solution to test its UV absorption spectrum; the probe was dissolved in acetonitrile solution at a concentration of 10 μM, and different anions (100 μM of fluoride ion, dihydrogen phosphate, acetate, bromide ion, hydrogen sulfate root, chlorate, iodide, chloride, nitrate) solution, test the change of fluorescence emission spectrum. Figure 1-Figure 7 It shows that fluorescent molecular probes have high sensitivity to fluoride ions in the ultraviolet absorption spectrum and fluorescence emission spectrum. After adding fluoride ions, the ultraviolet absorption spectrum is obviously red-shifted from 315 nm to 410 nm. When excited at 410 nm, the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com