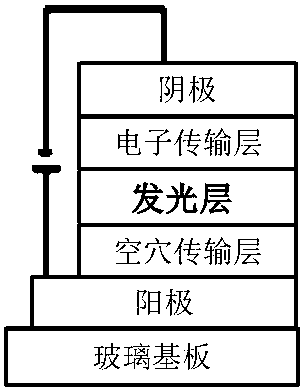
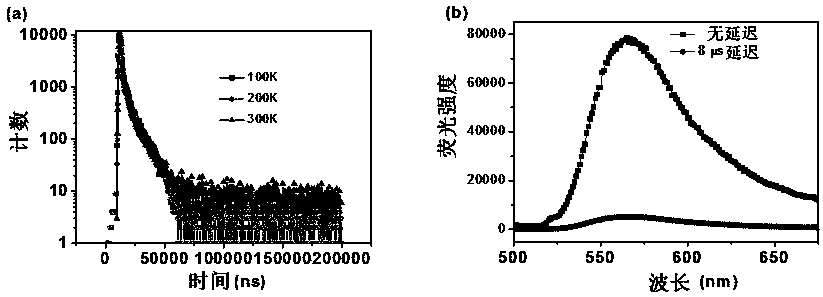
Malononitrile-substituted aryl anthracene-phenanthrene organic electroluminescent material and preparation method and application thereof
A technology of luminescent and luminescent dyes, which is applied in the direction of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems that it is difficult to have high exciton utilization rate and high fluorescence radiation efficiency at the same time, so as to improve device performance, external appearance and so on. The effect of good quantum efficiency and high power efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]
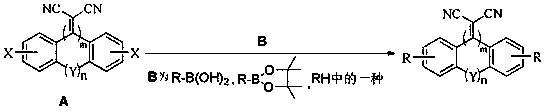
[0047] In a 250mL round bottom flask, add 2,2'-(3,6-dibromophenanthrene-9,10-dimethylene)malononitrile, 4-diphenylaminophenylboronic acid, Pd(PPh 3 ) 4 , vacuumize and blow argon gas. Add an oxygen-free mixed solvent (toluene, ethanol, saturated Na 2 CO 3solution), stirred and heated to reflux for 12 h. Sampling point plate, the reaction is complete, stop heating. Use saturated NH 4 The reaction was quenched with Cl solution and extracted three times with dichloromethane (DCM). Combine the organic phases, using anhydrous Na 2 SO 4 Dry, filter with suction, spin the filtrate to dryness, and separate by column chromatography to obtain the target product.
[0048] Elemental analysis structure (molecular formula: C 56 h 34 N 6 ): theoretical value: C 85.04; H 4.33; N 10.63, tested value: C 85.03; H 4.30; N 10.67. The relative molecular mass obtained by mass spectrometry is: 790.29.
Embodiment 2
[0050]
[0051] The preparation method of Example 2 is the same as that of Example 1, except that 4-diphenylaminophenylboronic acid is replaced with 4-(N-carbazolyl)phenylboronic acid.
[0052] Elemental analysis structure (molecular formula: C 56 h 30 N 6 ): Theoretical: C 85.48; H 3.84; N 10.68, tested: C 85.40; H 3.81; N 10.79. The relative molecular mass obtained by mass spectrometry is: 786.95.
Embodiment 3
[0054]
[0055] The preparation method of Example 3 is the same as that of Example 1, except that 4-diphenylaminophenylboronic acid is replaced with 9,9-diphenylfluorene borate.
[0056] Elemental analysis structure (molecular formula: C 70 h 40 N 4 ): theoretical value: C 89.72; H 4.30; N 5.98, tested value: C 89.71; H 4.27; N 6.02. The relative molecular mass obtained by mass spectrometry is: 936.35.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com