Thermal activation delayed fluorescence material based on dibenzothiophene sulfone
A technology of heat-activated delayed and fluorescent materials, applied in the direction of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of few types and low luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
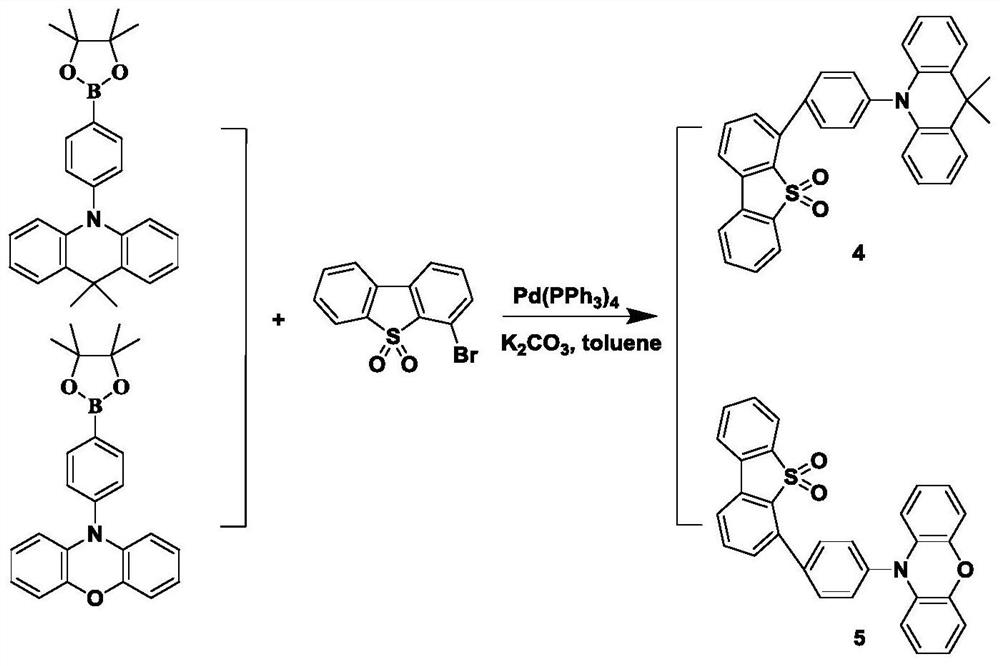
[0020] The target luminescent molecular structure of the present embodiment is
[0021] Refer figure 1 and figure 2 The synthesis route includes the following steps:
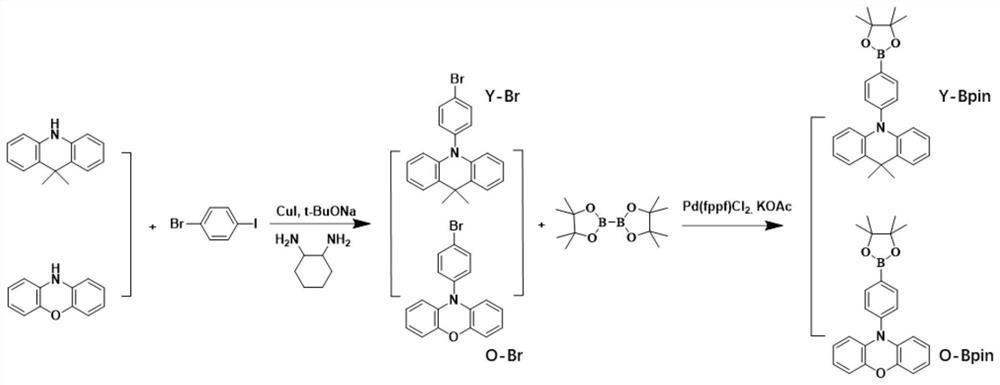
[0022] The first step: 9,9-dimethyl-9,10-dihydrridine (1 Equiv), 2-bromo-5-iodo (1 Equiv), copper iodide (0.02 equiv) and Sodium tert-butyl alcohol (2 equiv) is added to the reaction tube, and the nitrogen is drawn three times, and the mixture is stirred under nitrogen protection to add 50 ml of 1,4-dioxane (solvent). After the material is dissolved, 1,2-diaphrafonyl ring is added. Alkane (coordinator). The mixture was stirred at 110 ° C for 6 h. After cooling to room temperature, 100 ml of water was added to the reaction mixture and extracted with dichloromethane to obtain an organic layer. Finally, the organic layer was concentrated to obtain a crude product, separated by silica gel column chromatography to give a white solid product Y-BR with a yield of 70%.
[0023] Step 2: Y-BR (1 Equiv.), Combined boronic aci...
Embodiment 2
[0026] The target luminescent molecular structure of the present embodiment is:
[0027] Refer figure 1 and figure 2 The synthesis route includes the following steps:
[0028] The first step: Phenoxazine (1 Equivoxyl), 2-bromo-5-iodoenzene (1 Equiv.), Copper iodide (0.02 equiv.) And tert-butanol sodium (2 equiv.), The nitrogen was drawn three times, and 50 ml of 1,4-dioxane (coated 1,2-diaminocyclohexane) was added under nitrogen protection. The mixture was stirred at 110 ° C for 6 h. After cooling to room temperature, 100 ml of water was added to the reaction mixture and extracted with dichloromethane to obtain an organic layer. Finally, the organic layer was concentrated to obtain a crude product, separated by silica gel column chromatography to give a white solid product O-BR with a yield of 65%.
[0029] Step 2: O-BR (1 Equiv.), Which is the frequency of boronic acid-band, 1,1-di (diphenylphosphine) diomorite palladium (II) (0.02equiv. Potassium acetate (1.5 equiv.) Is dissol...
Embodiment 3
[0032] The target luminescent molecular structure of the present embodiment is:
[0033] Refer figure 2 The synthesis route includes the following steps:
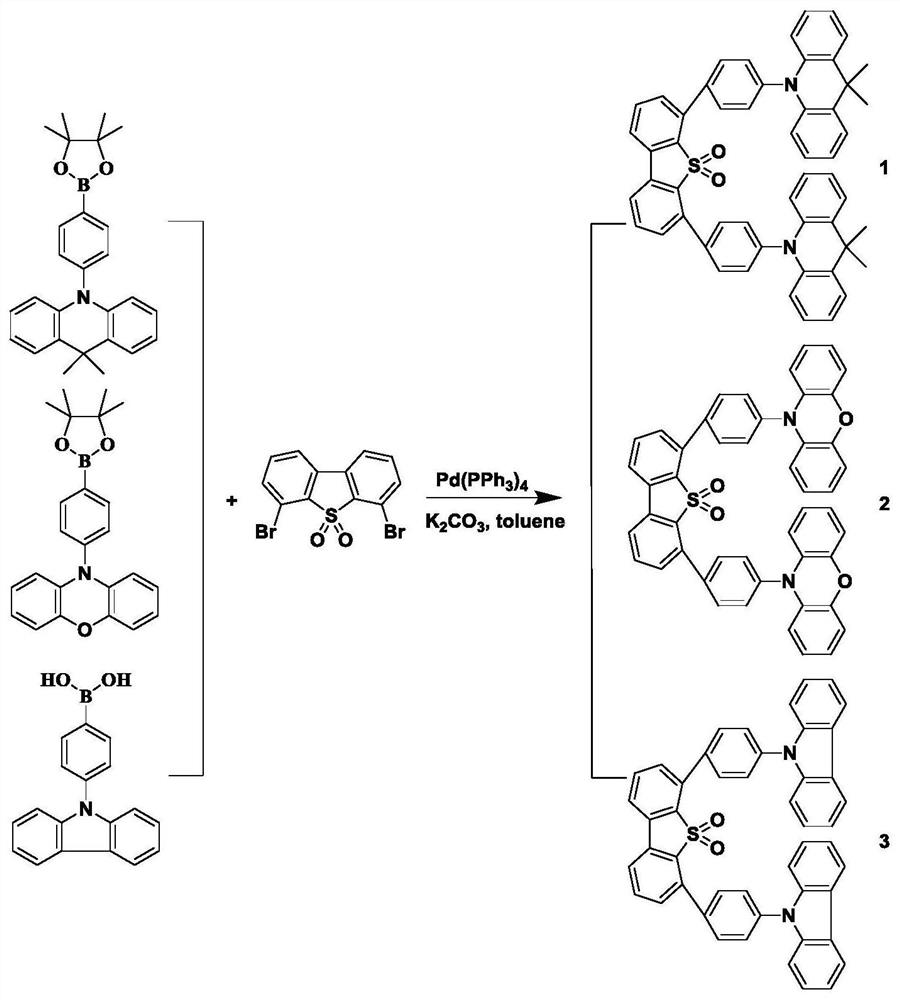
[0034] Carbazole boric acid (2.3 equiv.), 4,6-dibromo-diphenylene-5,5-dioxide (1 Equiv), four (triphenylphosphine) palladium (0.1equiv), 50 ml of toluene And 25 ml of potassium carbonate (concentration of 2 mol / L) was stirred at 120 ° C for 24 h. After cooling to room temperature, 100 ml of water was added to the reaction mixture and extracted with dichloromethane to obtain an organic layer. Finally, the organic layer was concentrated to obtain a crude product, separated by silica gel column chromatography to obtain a target luminescent molecule 3, and the yield was 80%. The nuclear magnetic table data is: 1 H NMR (400MHz, CDCL 3 : δ (PPM) 8.15 (D, 4H), 7.99 (D, 4H), 7.94 (D, 2H), 7.78 (T, 2H), 7.72 (D, 4H), 7.58 (DD, 6H), 7.42 ( T, 4H), 7.29 (t, 4h).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com