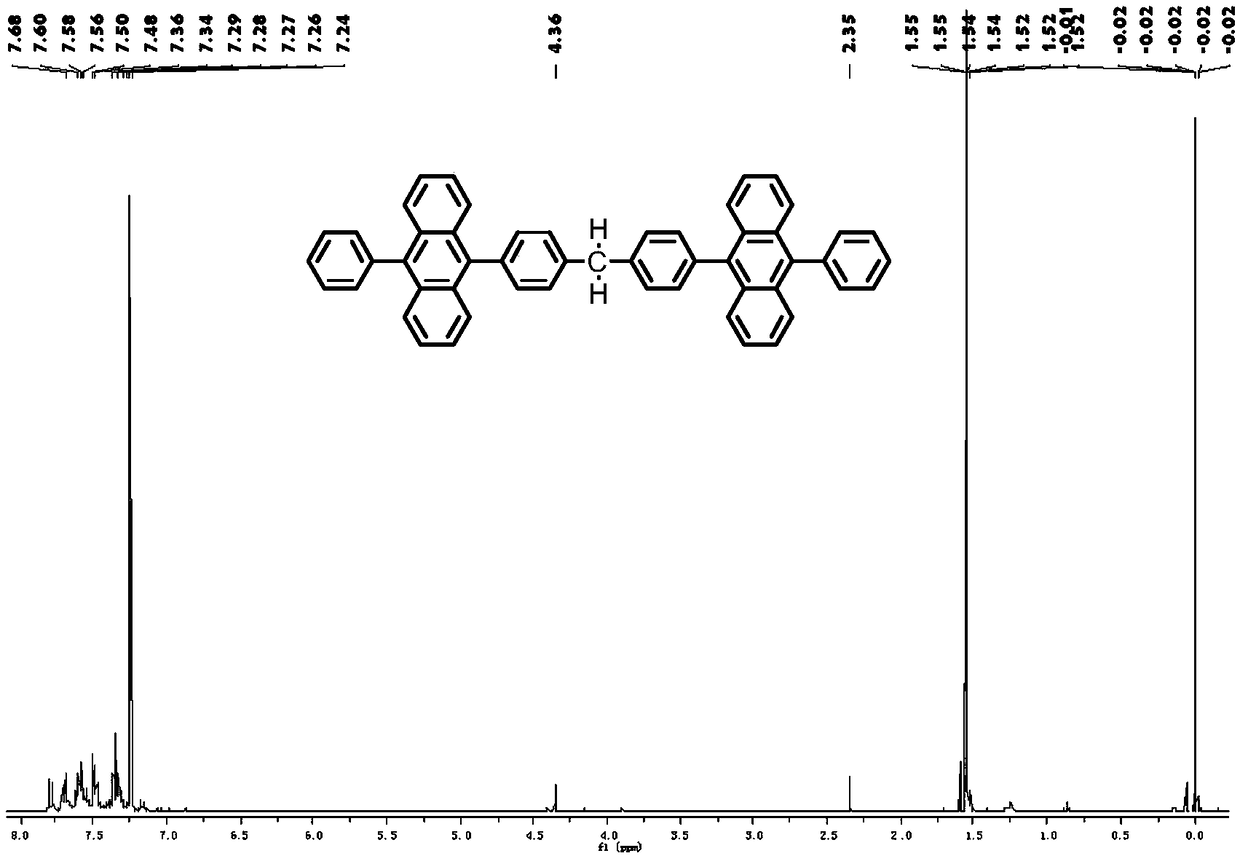
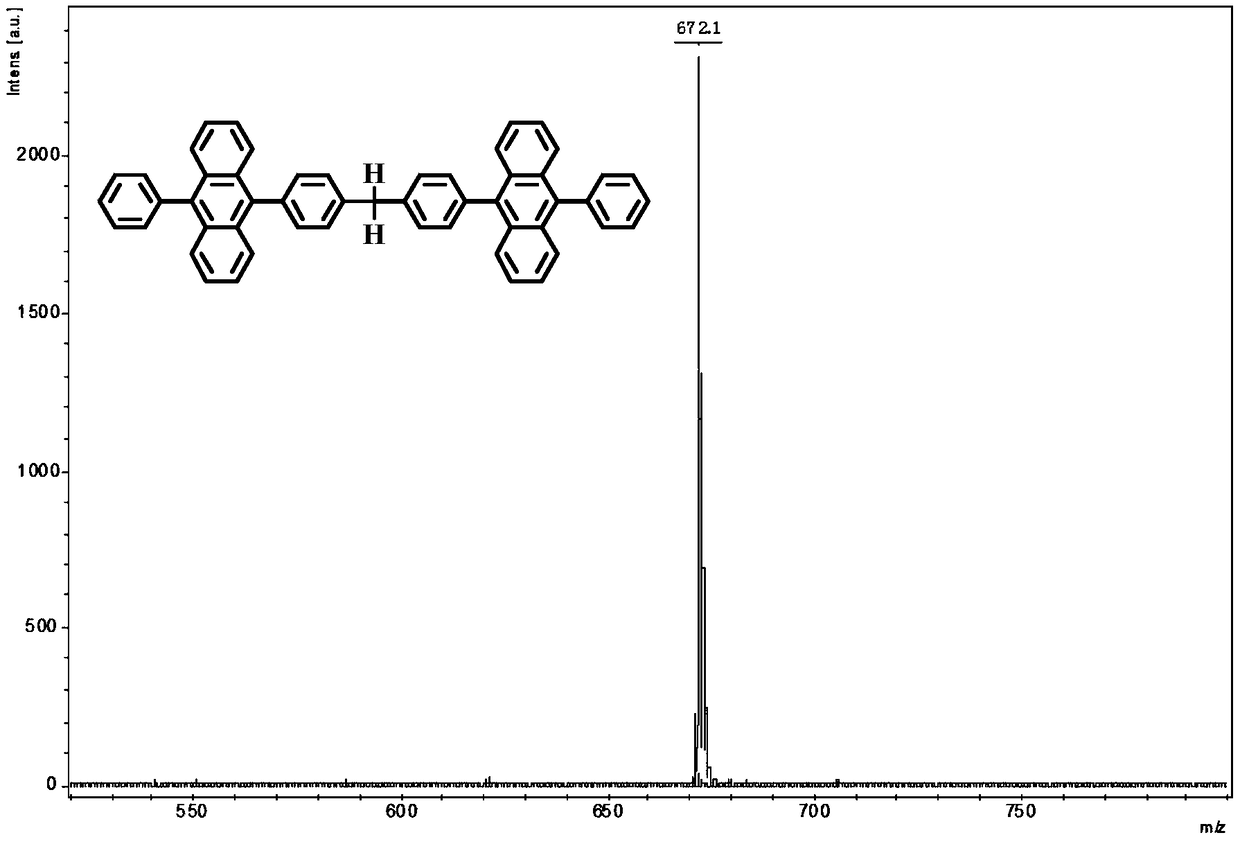
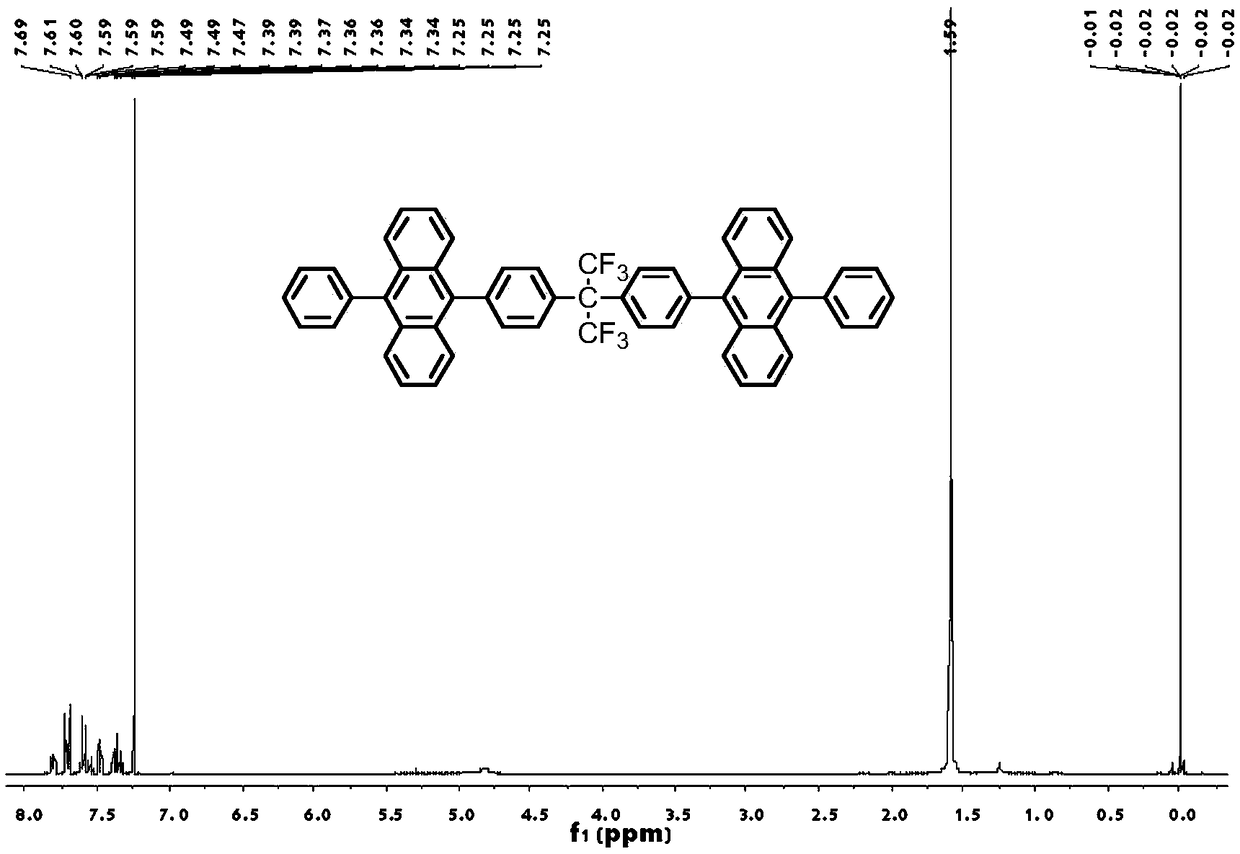
Organic blue fluorescent material and preparation method and application thereof
A blue fluorescent and organic technology, applied in the field of organic blue fluorescent materials and its preparation, can solve the problems of low yield rate of OLED production line, high production cost of vacuum evaporation process, difficult synthesis, etc., to achieve deep blue light emission, suppress π-π stacking action, effect of reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] ①Add 2.60g of 9-bromoanthracene, 1.83g of phenylboronic acid, 13.82g of potassium carbonate (add 30mL of distilled water to make a 2.0M solution), 100mL of toluene, and 30mL of ethanol into the reaction flask, then add 0.58g of tetrakis(triphenylphosphine )palladium. Then vacuumize the system, and reflux at 100° C. for 12 hours under the protection of nitrogen. After the reaction, the product was obtained by toluene extraction, rotary evaporation, column chromatography (eluent: n-hexane), and recrystallization (n-hexane / toluene=4:1). Yield 86%.
[0057] ②Bromination of 9-benzoanthracene: Add 2.15g of 9-benzoanthracene, 100mL of DMF, and 1.80g of NBS into the reaction flask, then vacuumize the system, and react under nitrogen protection at 85°C for 1 hour. After the reaction, the product was washed with methanol and suction filtered to obtain the product 9-bromo-10-benzanthracene. Yield 85%.
[0058] ③9-Bromo-10-Benzanthracene Boronate: Add 1.40g of 9-Bromo-10-Benzan...
Embodiment 2
[0062] ① Add 3.50g of 9-bromoanthracene, 2.52g of phenylboronic acid, 18.82g of potassium carbonate (add 45mL of water to form a solution), 136mL of toluene, and 45mL of ethanol into the reaction flask, and finally add 0.82g of tetrakis(triphenylphosphine) palladium. The system was evacuated and refluxed at 105° C. for 18 hours under nitrogen protection. After the reaction, the product was obtained by toluene extraction, rotary evaporation, column chromatography (eluent: n-hexane), and recrystallization (n-hexane / toluene=4:1). Yield 86%. ②9-Benzanthracene bromination: Add 3.50 g of 9-Benzanthracene, 130 mL of DMF, and 2.94 g of NBS into the reaction flask, then vacuumize the system, and react at 88°C for 1.5 h under the protection of nitrogen. After the reaction, the product was washed with methanol and filtered with suction to obtain the product 9-bromo-10-benzanthracene with a yield of 87%. ③Boronization of 9-bromo-10-benzanthracene: Add 2 g of 9-bromo-10-benzanthracene, ...
Embodiment 3
[0064] ①Add 5.14g of 9-bromoanthracene, 3.66g of phenylboronic acid, 27.64g of potassium carbonate (add 60mL of water to form a solution), 200mL of toluene, and 60mL of ethanol into the reaction flask, and finally add 1.16g of tetrakis(triphenylphosphine)palladium . Then the system was evacuated and refluxed at 110° C. for 24 hours under the protection of argon. After the reaction, the product was obtained by toluene extraction, rotary evaporation, column chromatography (eluent: n-hexane), and recrystallization (n-hexane / toluene=4:1). Yield 86%. ②9-Benzanthracene bromination: Add 4.29g of 9-benzanthracene, 200mL of DMF, and 3.6g of NBS into the reaction flask, then vacuumize the system, and react at 90°C for 2 hours under the protection of argon. After the reaction was completed, the product was washed with methanol and suction filtered to obtain the product 9-bromo-10-benzanthracene with a yield of 77%. ③9-Bromo-10-benzoanthracene boronate: Add 2.80g of 9-bromo-10-benzoant...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal decomposition temperature | aaaaa | aaaaa |
| Square resistance | aaaaa | aaaaa |
| Maximum current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com