A kind of d-a type organic blue fluorescent material and its preparation method and application
A blue fluorescence, D-A technology, applied in the preparation of organic compounds, luminescent materials, organic chemistry, etc., can solve the problems of difficult purification, low efficiency of OLED devices, difficult synthesis, etc., to achieve inhibition of π-π stacking and high luminous quantum efficiency , The effect of simplifying the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
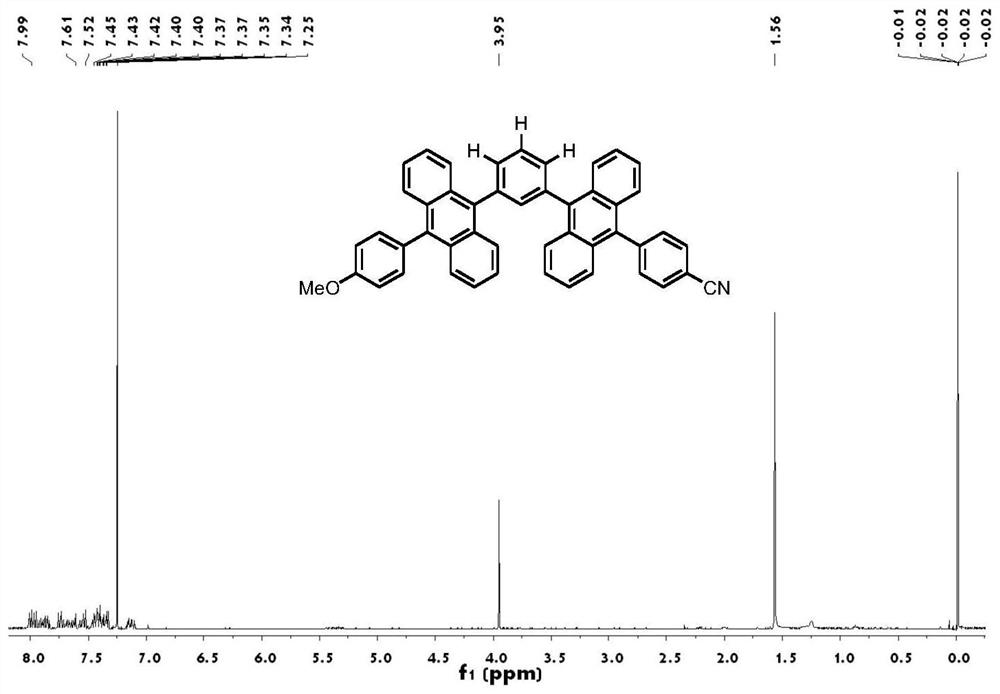
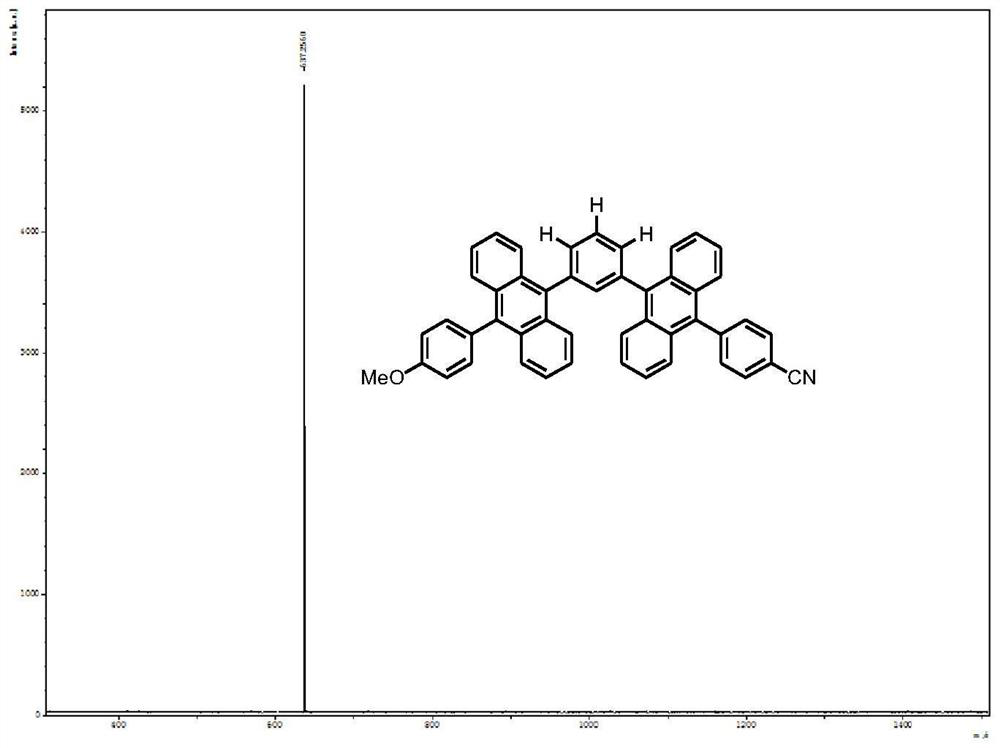
[0068] Step 1, synthesis of groups with electron-donating properties: ① Add 5 mmol of 9-bromoanthracene, 7.5 mmol of 4-methoxyphenylboronic acid, 50 mmol of potassium carbonate solution (make a solution with 15 mL of distilled water), 50 mL of toluene, and 15 mL of ethanol into the reaction bottle, and finally add 0.25mmol tetrakis(triphenylphosphine)palladium. Then the system was evacuated and refluxed at 100° C. for 12 hours under the protection of nitrogen. After the reaction, extraction, rotary evaporation, column chromatography (eluent: n-hexane / dichloromethane=4:1), recrystallization to obtain 9-(4-methoxy)benzanthracene. Yield 86%. ②Add 2.50mmol of 9-(4-methoxy)benzoanthracene, 40mL of N,N-dimethylformamide (DMF), and 3mmol of N-bromosuccinimide (NBS) into the reaction flask, and then The system was evacuated, and reacted at 85° C. for 1 hour under the protection of nitrogen. Washing with methanol and suction filtration gave the product 9-bromo-10-(4-methoxy)benzanth...
Embodiment 2
[0072] Step 1 Synthesize groups with electron-donating properties: ①Add 10mmol of 9-bromoanthracene, 15mmol of 4-methoxyphenylboronic acid, 100mmol of potassium carbonate solution (make a solution with 30mL of distilled water), 136mL of toluene, and 30mL of ethanol into the reaction flask, Finally 0.5 mmol of tetrakis(triphenylphosphine)palladium was added. Then vacuumize the system, under the protection of nitrogen, reflux at 105 for 18 hours. After the reaction, extraction, rotary evaporation, column chromatography (eluent: n-hexane / dichloromethane=4:1), recrystallization to obtain 9-(4-methoxy)benzanthracene. Yield 90%. ②Add 5mmol of 9-(4-methoxy)benzanthracene, 75mL of N,N-dimethylformamide (DMF), and 6mmol of N-bromosuccinimide (NBS) into the reaction flask, and then Vacuum, under the protection of nitrogen, react at 88°C for 1.5 hours. Washing with methanol and suction filtration gave the product 9-bromo-10-(4-methoxy)benzanthracene with a yield of 89%.
[0073]Step ...
Embodiment 3
[0076] Step 1 Synthesis of groups with electron-donating properties: ①Add 20mmol of 9-bromoanthracene, 30mmol of 4-methoxyphenylboronic acid, 200mmol of potassium carbonate solution (make a solution with 60mL of distilled water), 200mL of toluene, and 60mL of ethanol into the reaction flask, Finally 1 mmol of tetrakis(triphenylphosphine)palladium was added. Then the system was evacuated and refluxed at 110° C. for 24 hours under the protection of nitrogen. After the reaction, extraction, rotary evaporation, column chromatography (eluent: n-hexane / dichloromethane=4:1), recrystallization to obtain 9-(4-methoxy)benzanthracene. Yield 89%. ②Add 10mmol of 9-(4-methoxy)benzanthracene, 150mL of N,N-dimethylformamide (DMF), and 12mmol of N-bromosuccinimide (NBS) into the reaction flask, and then Vacuumize and react at 90°C for 2 hours under nitrogen protection. Washing with methanol and suction filtration gave the product 9-bromo-10-(4-methoxy)benzanthracene with a yield of 90%.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
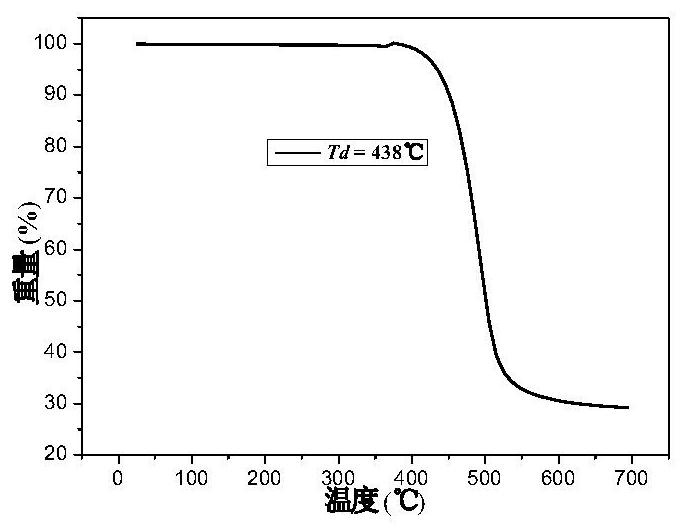
| thermal decomposition temperature | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com