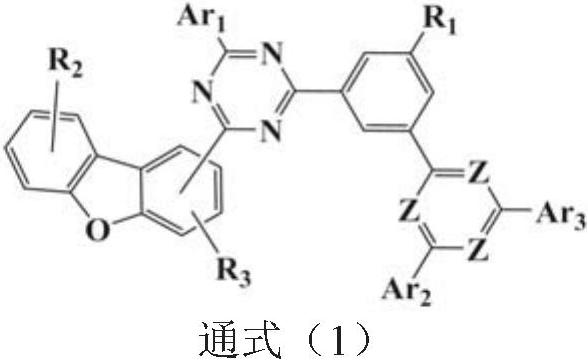
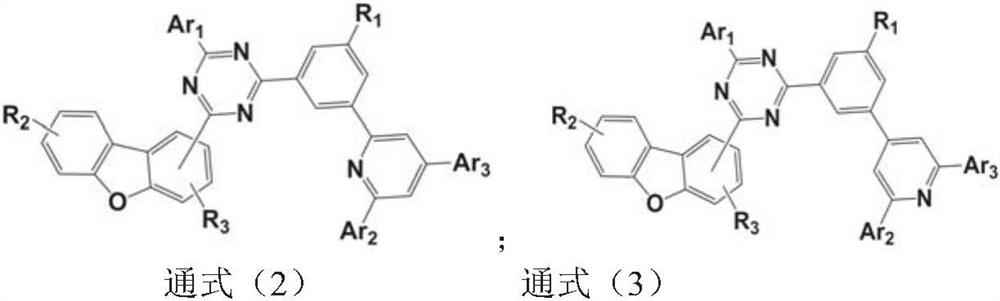
Phenylpyridine-containing compound, preparation method, organic electroluminescent device and display element
A technology of phenylpyridine and compounds, which is applied in the field of semiconductor materials, can solve the problems of material phase separation or decomposition, insufficient heat resistance stability, electron tolerance defects, etc., and achieve high glass transition temperature and good electron mobility , Improve the photoelectric performance and the effect of device life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
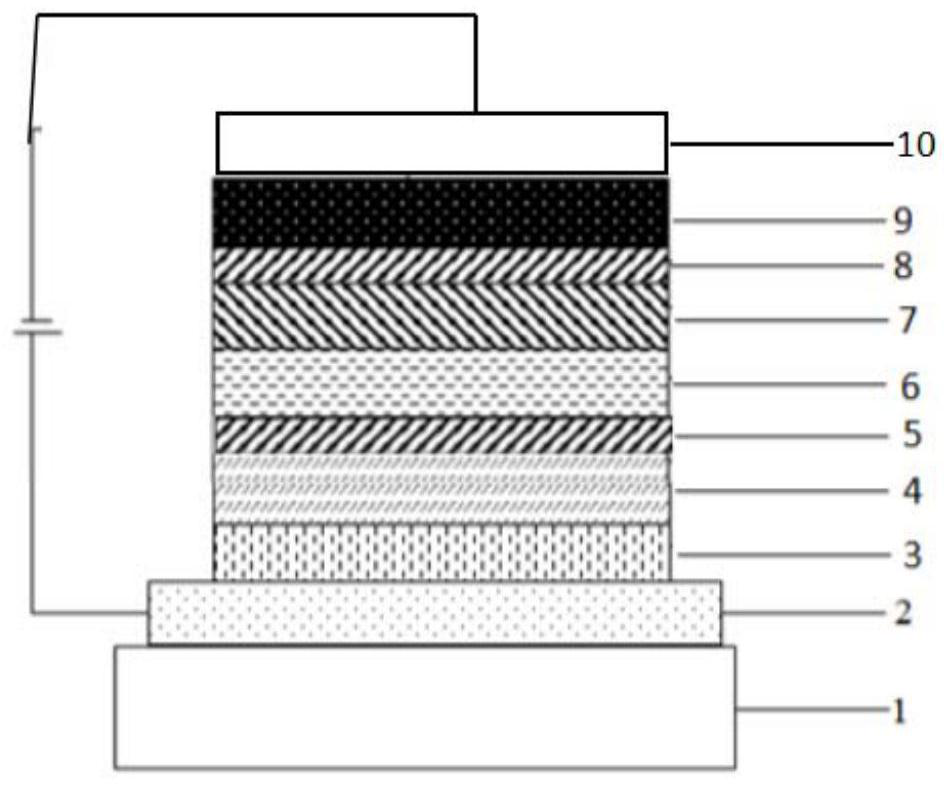
Image
Examples
Embodiment 1
[0108] Embodiment 1: the synthesis of compound 1:
[0109]
[0110] In the three-necked flask, nitrogen was introduced, 0.022mol of intermediate A-1, 100ml of DMF, 0.02mol of intermediate B-1, 0.0002mol of palladium acetate were added, stirred, and then 0.03mol of K 3 PO 4 Aqueous solution, heating and reflux reaction for 16 hours, sampling plate, the reaction is complete. Cool naturally, pour the reaction solution into a 500ml beaker, add 250ml distilled water, and carry out mechanical stirring for 20min, then the mixed solution is suction filtered, the filter cake is rinsed twice with 200ml distilled water, and then rinsed with 100ml ethanol to obtain White solid powder. Finally, the white solid powder was purified by a silica gel column with an eluent of ethyl acetate:petroleum ether=1:4 to obtain Compound 1 with a HPLC purity of 99.14% and a yield of 65.7%. Elemental analysis structure (molecular formula C 44 h 28 N 4 O): theoretical value C, 84.06; H, 4.49; N, 8....
example 3
[0138] An organic electroluminescent device was prepared in the same manner as in Device Example 1, except that the compounds shown in Table 2 were used to replace the electron-transport compound 1 in Device Example 1, wherein the ratio of the electron-transport compound and Liq was 1:1, control the evaporation rate as and The specific device structure is shown in Table 4.
[0139] After the fabrication of the electroluminescence device was completed according to the above steps, the efficiency data and light decay lifetime of the device were measured, and the results are shown in Table 5. The molecular structure formula of the relevant material is as follows:
[0140]
[0141] The structures of comparative compounds ET-1, ET-2 and ET-3 are given above. All the above materials are commercially available.
[0142] Table 4
[0143]
[0144]
[0145]
[0146] III. Device Test Example
[0147]The devices prepared in II were tested to test their driving voltage,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com