Triphenyl amine dyes for dye-sensitized solar cells
A technology for solar cells and dye sensitization, applied in the field of dye-sensitized solar cells, can solve the problems of inability to popularize and expensive sensitizers, etc., and achieve the effects of excellent optoelectronic performance, low cost, and rich research content.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the synthesis of triphenylamine-based dyes
[0026] The synthetic route is as follows:
[0027]
[0028] In a three-necked flask equipped with a drying tube, add 5.11 g (21.5 mmol) of triphenylamine and 25 mL of dry N,N-dimethylformamide (DMF), and cool in an ice-salt bath. Slowly add POCl dropwise at 0°C 3 21 mL (35.16 g, 269.4 mmol). After the dropwise addition was completed, the temperature was raised to 90-105° C., and the reaction was stirred for 8 hours. Cool to room temperature after the reaction, pour the crude product into 60 mL of ice water, add dropwise 20% NaOH aqueous solution to adjust the pH value to 6-8, stir, and filter to obtain a khaki solid. use CH 2 Cl 2 Dissolve the solid crude product, add anhydrous NaSO 4 Let dry overnight. The solvent was evaporated by rotary evaporation and purified by column chromatography (petroleum ether::dichloromethane:ethyl acetate=7:3:1 as eluent, R f =0.4), to get earthy yellow substance triphe...
Embodiment 2
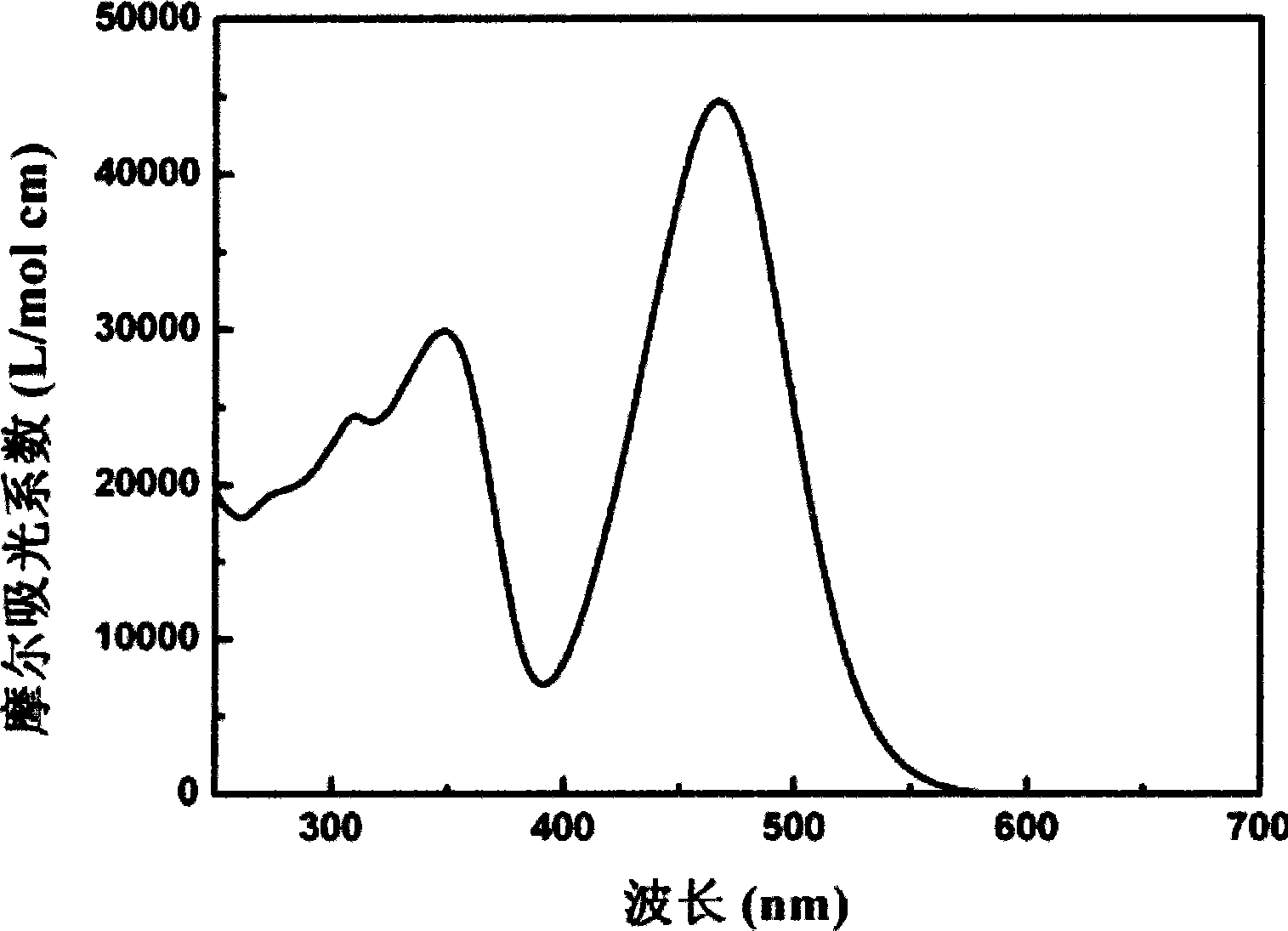
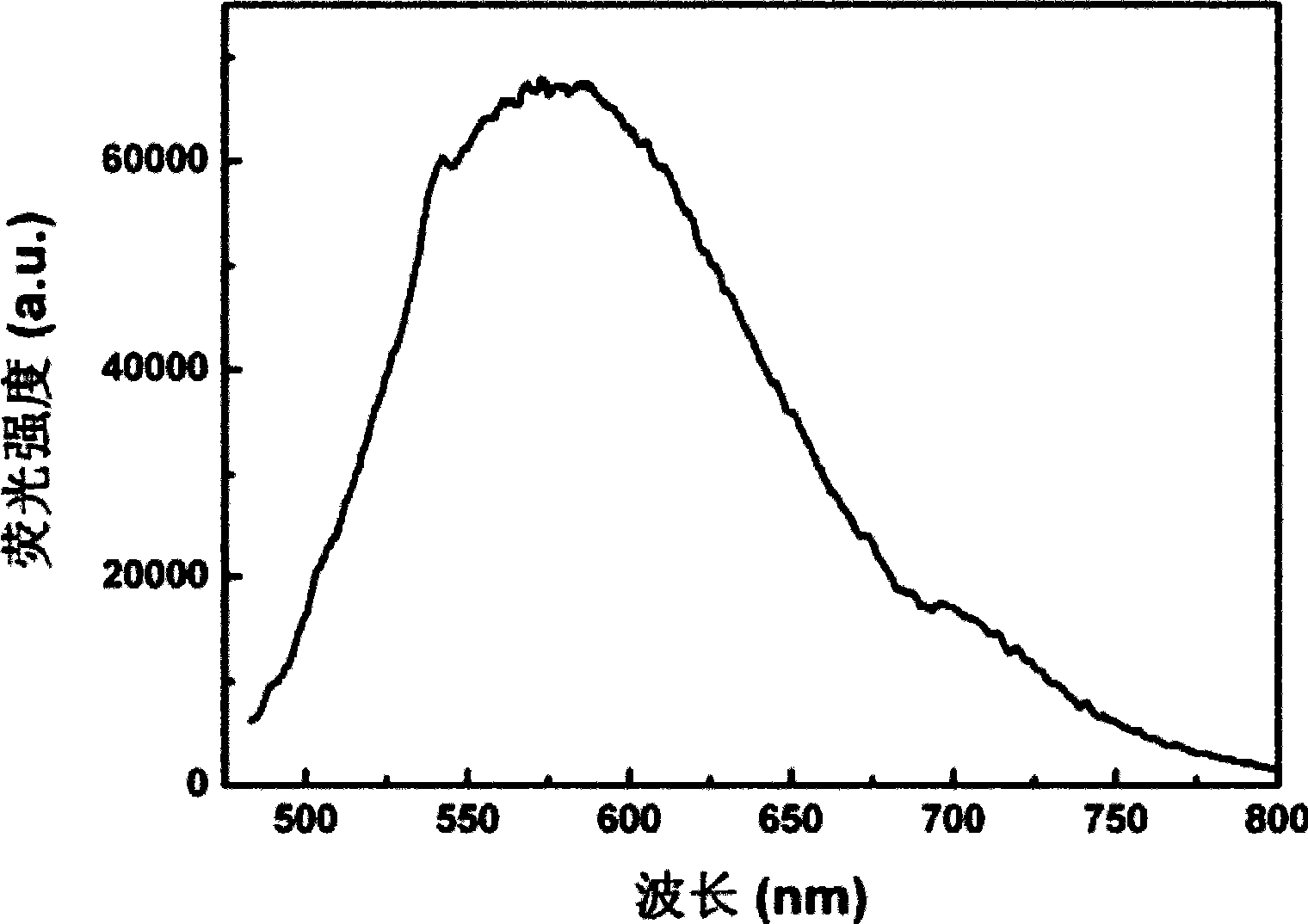
[0031] Embodiment 2: to the test of the ultraviolet-visible absorption spectrum / fluorescence spectrum of dyestuff in embodiment 1
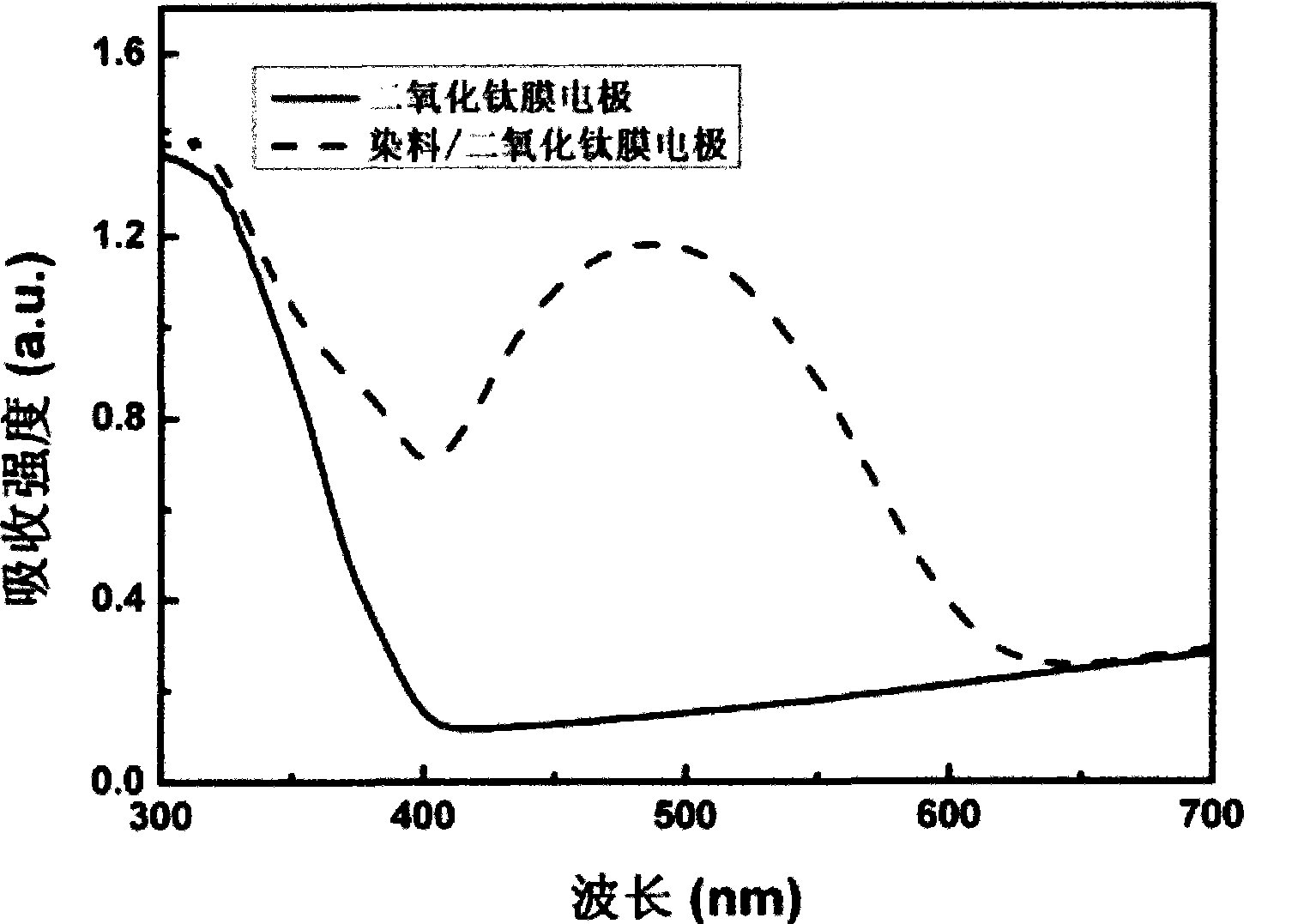
[0032] The triphenylamine dye that embodiment 1 prepares is mixed with 5 * 10 -5 The methanol solution of mol / L adopts Japan Jasco V-550 ultraviolet-visible spectrophotometer to carry out the test of absorption spectrum (see Figure 1a ). Carry out the fluorescence test by adopting American Cary Eclipse fluorescence spectrophotometer (see Figure 1b ). The ultraviolet absorption spectrum of the triphenylamine-based dye-sensitized titanium dioxide film electrode (see figure 2 ) test using the same instrument. The test of the adsorption amount of triphenylamine-based organic dyes adsorbed on the titanium dioxide film is that the dye-sensitized TiO 2 The nanocrystalline film was soaked in 10mL 0.01mol / L sodium hydroxide methanol solution overnight, and the absorbance of the solution was measured after the dye was completely desorbed; the adsorpt...
Embodiment 3
[0036] Embodiment 3: to the estimation of electrochemical test and HOMO and LUMO energy level of the dyestuff in embodiment 1
[0037] Differential pulse voltammetry (DPV) was used to test the redox potential of the dye in the ground state, and the instrument used was the PARSTAT 2273 electrochemical workstation in the United States. The electrolyte used in the test is acetonitrile solvent, with 0.1mol / L tetrabutylammonium perchlorate as the supporting electrolyte. The test uses a three-electrode system: glassy carbon electrode as the working electrode; platinum wire electrode as the counter electrode; Ag / AgNO 3 (acetonitrile) as a reference electrode, the calibration of which is carried out by measuring the redox potential of ferrocene in acetonitrile. The measured redox potential was converted to a value relative to a standard hydrogen electrode (vs. NHE), which determined the HOMO level of the dye. The LUMO energy level of the dye is given by the formula LUMO=HOMO—E 0-0 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com