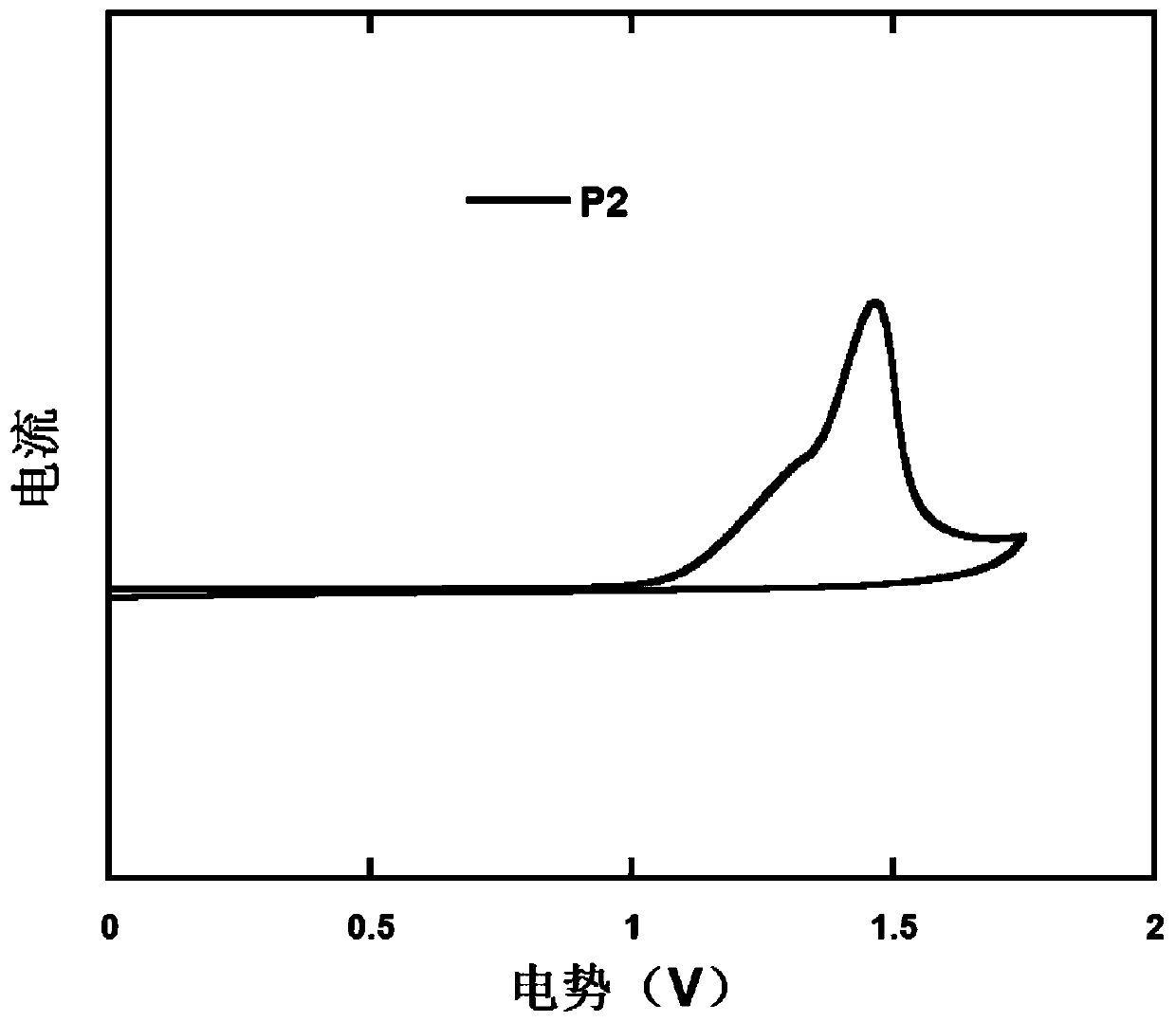
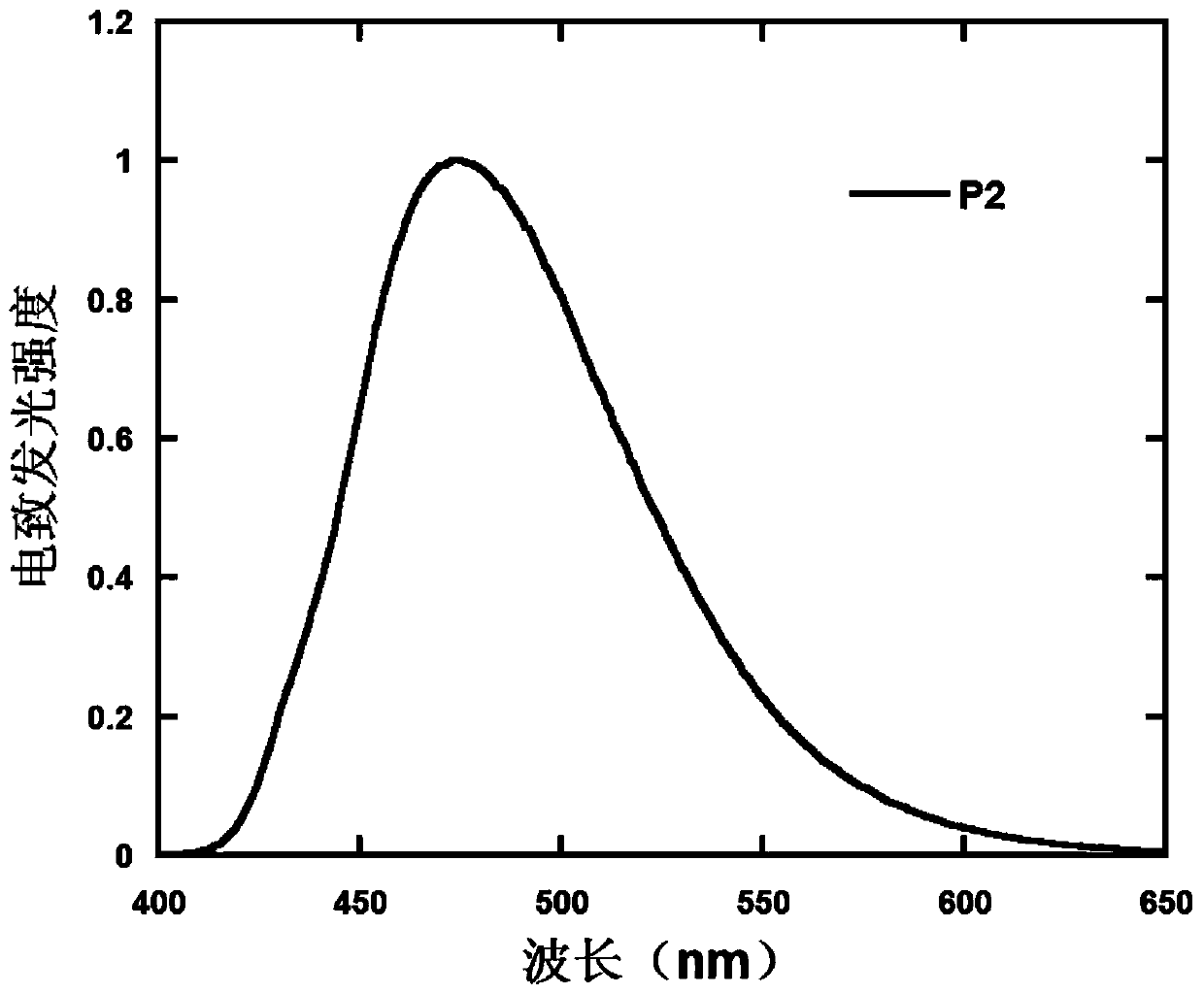
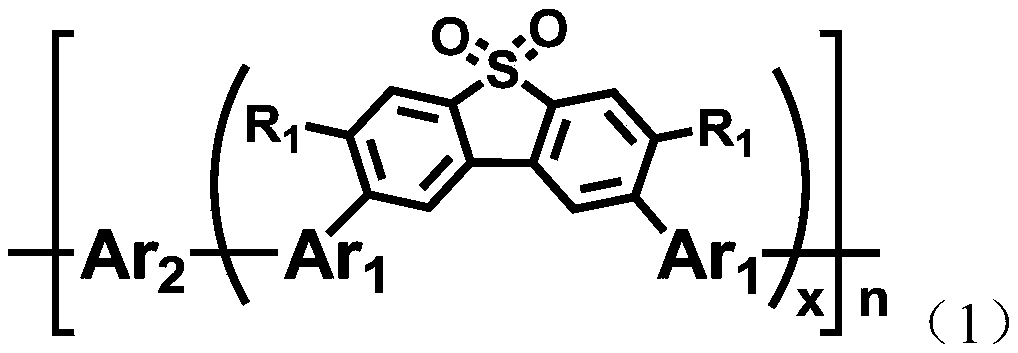
D-a type polymer containing s, s-dioxy-dibenzothiophene in the main chain and its preparation method and application
A dibenzothiophene and polymer technology, applied in the field of D-A polymer and its preparation, can solve problems such as limiting electroluminescence efficiency, and achieve the effects of benefiting device efficiency, increasing the generation ratio, and promoting the crossing process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the preparation of compound M1
[0035] (1) Preparation of compound 1
[0036] Add 2,8-dibromo-S, S-dioxy-dibenzothiophene (3.74g, 10mmol), aniline (2.79g, 30mmol), sodium tert-butoxide (4.81g, 50mmol) into a 100ml two-necked flask, Tris(dibenzylideneacetone)dipalladium (458 mg, 0.5 mmol), tri-tert-butylphosphine (101 mg, 0.5 mmol) and 50 ml of toluene were heated to 100° C. for 12 hours under nitrogen. After the reaction was completed, the product was extracted with dichloromethane, the organic phase was washed with saturated aqueous sodium chloride solution, the solvent was evaporated under reduced pressure, and the crude product was eluted with a mixed solvent of petroleum ether:dichloromethane=3:1 (v / v) Purified by solvent column chromatography to obtain 1.60 g of white solid with a yield of 40%, (mass spectrum: 398.2).
[0037] (2) Preparation of compound M1
[0038]Add compound 1 (398.5g, 10mmol), p-bromoiodobenzene (8.49g, 30mmol), sodium tert-bu...
Embodiment 2
[0041] Embodiment 2: the preparation of compound M2
[0042] Add 2,8-diiodo-S, S-dioxo-dibenzothiophene (4.68g, 10mmol), 3-bromocarbazole (6.15g, 25mmol), potassium carbonate (6.9g, 50mmol) into a 100ml two-necked flask ), cuprous iodide (95mg, 0.5mmol) and 50ml of toluene were heated to 100°C for 12 hours under nitrogen atmosphere. After the reaction was completed, the product was extracted with dichloromethane, the organic phase was washed with saturated aqueous sodium chloride solution, the solvent was evaporated under reduced pressure, and the crude product was eluted with a mixed solvent of petroleum ether:dichloromethane=4:1 (v / v) Purified by solvent column chromatography to obtain 2.59 g of white solid with a yield of 37%, (mass spectrum: 704.0).
[0043] The chemical reaction equation is shown in formula (3):
[0044]
Embodiment 3
[0046] Preparation of compound M3
[0047] Under a nitrogen atmosphere, add 3,6-dibromocarbazole (3.25g, 10mmol), 2-ethylbromohexane (2.90g, 15mmol), potassium carbonate (4.14g, 30mmol) to a 300ml two-necked flask ) and 150ml N,N-dimethylformamide were stirred and reacted at 80°C for 12 hours. After cooling, the product was extracted with dichloromethane, washed five times with saturated aqueous sodium chloride solution until the water layer was clear, distilled off under reduced pressure to remove dichloromethane, and the crude product was purified by column chromatography using petroleum ether as eluent to obtain a colorless transparent liquid 3.94 g, yield 90%, (mass spectrum: 437.1).
[0048] Preparation of compound M4
[0049] Under nitrogen protection, add M3 (4.37g, 10mmol), pinacol diborate (6.35g, 25mmol), [1,1'-bis(diphenylphosphino)ferrocene] into a 300ml two-necked flask Palladium dichloride (0.49g, 0.5mmol), potassium acetate (3.92g, 40mmol) and 120ml of dioxan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com