Method for preparing (Z)-5-amino-alpha-(ethoxy imino group)-1, 2, 4-thiadiazole-3-acetic acid
An ethoxyimino, thiadiazole technology, applied in the direction of organic chemistry and the like, can solve the problems of large environmental goals, many steps, long routes, etc., and achieves the effects of fewer synthesis steps, low cost and less pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
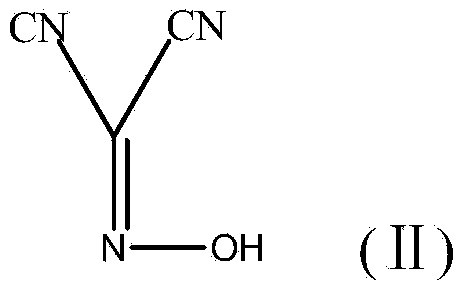
[0036] 1) Add 1 mol of malononitrile, 1 mol of water and 2 mol of glacial acetic acid into a three-necked flask, cool down to 0°C, add 4 mol of sodium nitrite, react at 0°C for 3 hours to obtain reaction product A, adjust the pH value of reaction product A 2. Then extract the reaction product A with 100g of ethyl acetate each time, and extract three times in total. Combine the three extraction phases and remove the solvent by rotary evaporation under reduced pressure, that is, in a water bath at 40°C and a rotary evaporator with a vacuum of 0.08MPa The solvent was removed in the middle to obtain a reddish-brown liquid, namely compound A;
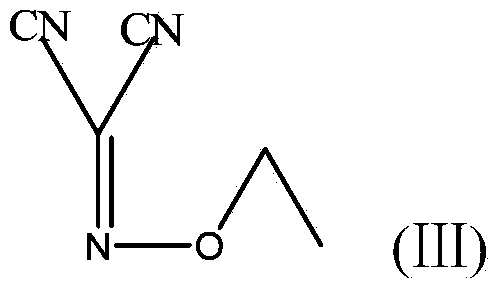
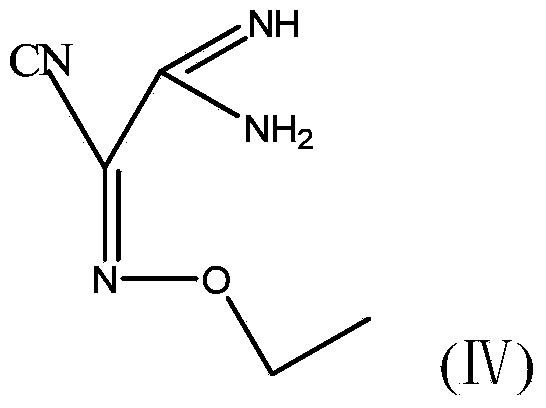
[0037] 2) Under the protection of argon, add 1 mol of compound A to the three-necked flask, cool down to 5°C, add 3 mol of triethylamine, stir for 1 hour, then add 2 mol of bromoethane, and then rise to room temperature to dissolve the bromoethane, and then React at 5°C for 3 hours to obtain the reaction product B, add 300g of water to the r...
Embodiment 2
[0042] 1) Add 1 mol of malononitrile, 2 mol of water and 3 mol of glacial acetic acid into the three-necked flask, cool down to 5°C, add 2 mol of sodium nitrite, and react at 5°C for 4 hours to obtain the reaction product A, adjust the pH value of the reaction product A 2. Then extract the reaction product A with 100g of ethyl acetate each time, and extract three times in total. After combining the three extraction phases, the solvent is removed by rotary evaporation under reduced pressure, that is, in a water bath at 30°C and a rotary evaporator with a vacuum of 0.07MPa The solvent was removed in the middle to obtain a reddish-brown liquid, namely compound A;
[0043] 2) Under the protection of neon gas, add 1 mol of compound A to the three-necked flask, cool down to 0°C, add 2 mol of triethylamine, stir for 2 hours, then add 3 mol of bromoethane, and then rise to room temperature to dissolve the bromoethane, and then React at 0°C for 3.5 hours to obtain the reaction product B,...
Embodiment 3
[0048] 1) Add 1 mol of malononitrile, 1.5 mol of water and 2.5 mol of glacial acetic acid into a three-necked flask, cool down to 2°C, add 3 mol of sodium nitrite, react at 2°C for 5 hours to obtain reaction product A, adjust the pH of reaction product A The value is 2, and then the reaction product A is extracted three times with 100g ethyl acetate at a time, and the extraction phases of the three times are combined and then the solvent is removed by rotary evaporation under reduced pressure, that is, in a water bath at 35°C and a vacuum of 0.06MPa. The solvent was removed in an evaporator to obtain a reddish-brown liquid, namely compound A;
[0049] 2) Under the protection of nitrogen, add 1 mol of compound A to the three-necked flask, cool down to 2°C, add 2.5 mol of triethylamine, stir for 1.5 h, then add 2.5 mol of diethyl sulfate, and then rise to room temperature to make diethyl sulfate Dissolve, then react at 2°C for 3 h to obtain reaction product B, add 300 g of water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com