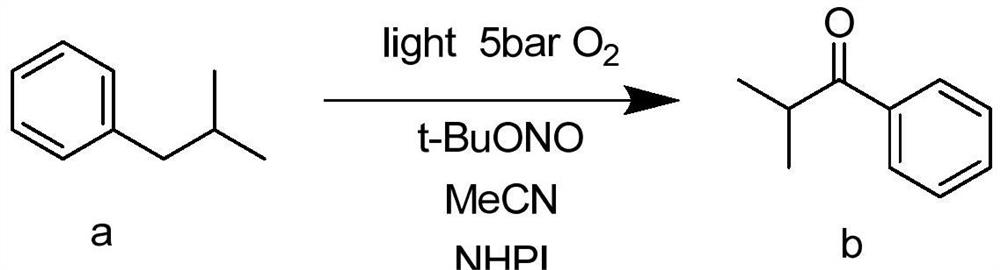
Method for preparing aromatic hydrocarbon alpha ketone carbonyl compound through continuous visible light catalytic molecular oxygen oxidation in micro-channel reactor
A technology of microchannel reactor and visible light, which is applied in the preparation of carbon-based compounds, organic compounds, chemical instruments and methods, etc., can solve the problems of not conforming to the principle of economical green, not suitable for obtaining, and high cost, so as to reduce energy and labor , low cost, and the effect of reducing emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
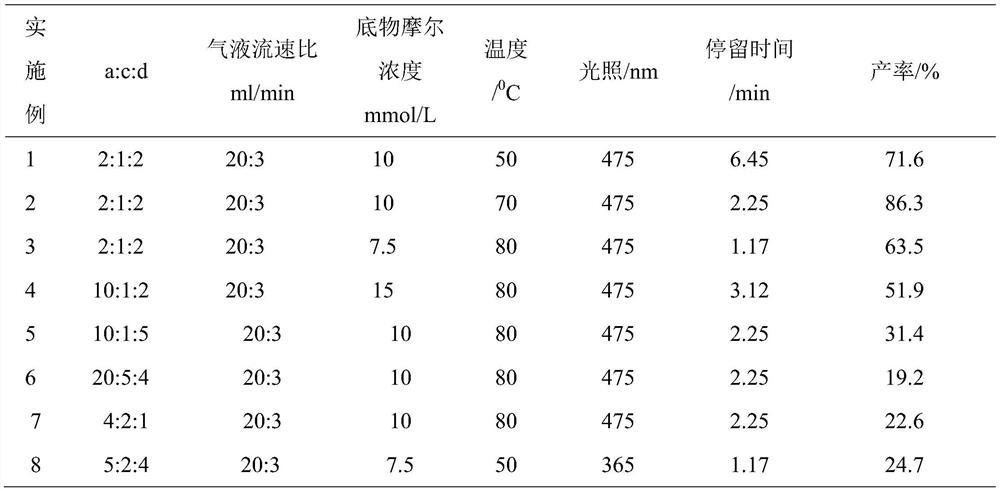
[0027] Add 0.67g of isobutylbenzene, 0.51g of tert-butyl nitrite, and 0.41g of N-hydroxyphthalimide into the test tube, and add 15ml of acetonitrile into the test tube, mix well in a constant temperature stirrer at 60°C and dissolve completely. The mixed solution is pumped into the microchannel reactor through the feed pump, and oxygen is introduced at the same time. The molar ratio of isobutylbenzene: N-hydroxyphthalimide: tert-butyl nitrite is 2:1: 2. The reaction pressure was controlled to be 5 bar, the light intensity was 475 nm, the temperature was 50° C., and the reaction time of the reactants in the microchannel reactor was 6.45 min to obtain isobutyrophenone with a comprehensive yield of 71.6%. The acetonitrile is refined and reused, and the residue is refined to recover the catalyst.
Embodiment 2
[0029] Add 0.67g of isobutylbenzene, 0.51g of tert-butyl nitrite, and 0.41g of N-hydroxyphthalimide into the test tube, and add 15ml of acetonitrile into the test tube, mix well in a constant temperature stirrer at 60°C and dissolve completely. The mixed solution is pumped into the microchannel reactor through the feed pump, and oxygen is introduced at the same time. The molar ratio of isobutylbenzene: N-hydroxyphthalimide: tert-butyl nitrite is 2:1: 2. The reaction pressure was controlled to be 5 bar, the light intensity was 475 nm, the temperature was 70° C., and the reaction time of the reactants in the microchannel reactor was 2.25 min to obtain isobutyrophenone with a comprehensive yield of 86.3%. The acetonitrile is refined and reused, and the residue is refined to recover the catalyst.
Embodiment 3
[0031] Add 0.67g of isobutylbenzene, 0.51g of tert-butyl nitrite, 0.41g of N-hydroxyphthalimide into the test tube, and add 20ml of acetonitrile into the test tube, mix well in a constant temperature stirrer at 60°C and dissolve completely. The mixed solution is pumped into the microchannel reactor through the feed pump, and oxygen is introduced at the same time. The molar ratio of isobutylbenzene: N-hydroxyphthalimide: tert-butyl nitrite is 2:1: 2. The reaction pressure was controlled to be 5 bar, the light intensity was 475 nm, the temperature was 80° C., and the reaction time of the reactants in the microchannel reactor was 1.17 min to obtain isobutyrophenone with a comprehensive yield of 63.5%. The acetonitrile is refined and reused, and the residue is refined to recover the catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com