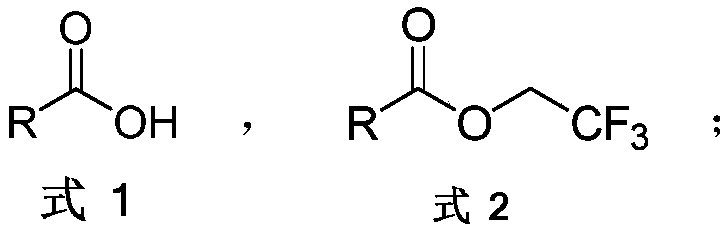
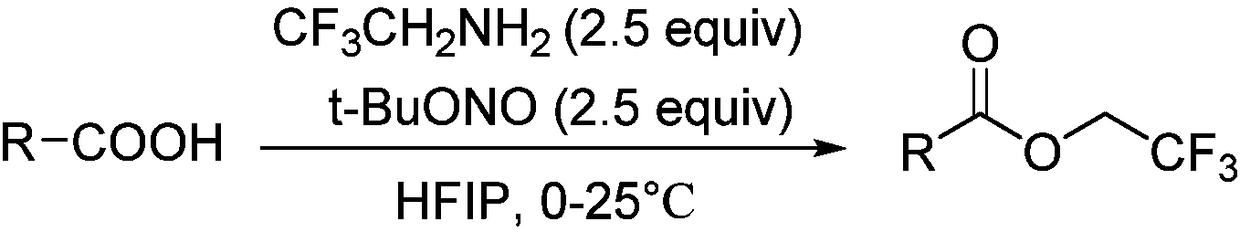Aromatic carboxylic acid trifluoroethyl ester compound and preparation method thereof
A technology for aromatic carboxylic acid trifluoroethyl ester and carboxylic acid trifluoroethyl ester, which is applied in the fields of preparing aromatic carboxylic acid trifluoroethyl ester compounds and preparing aromatic carboxylic acid trifluoroethyl ester compounds, and can solve harsh reaction conditions , high product yield, less by-products, etc., to achieve the effect of less side reactions, simple reaction operation and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
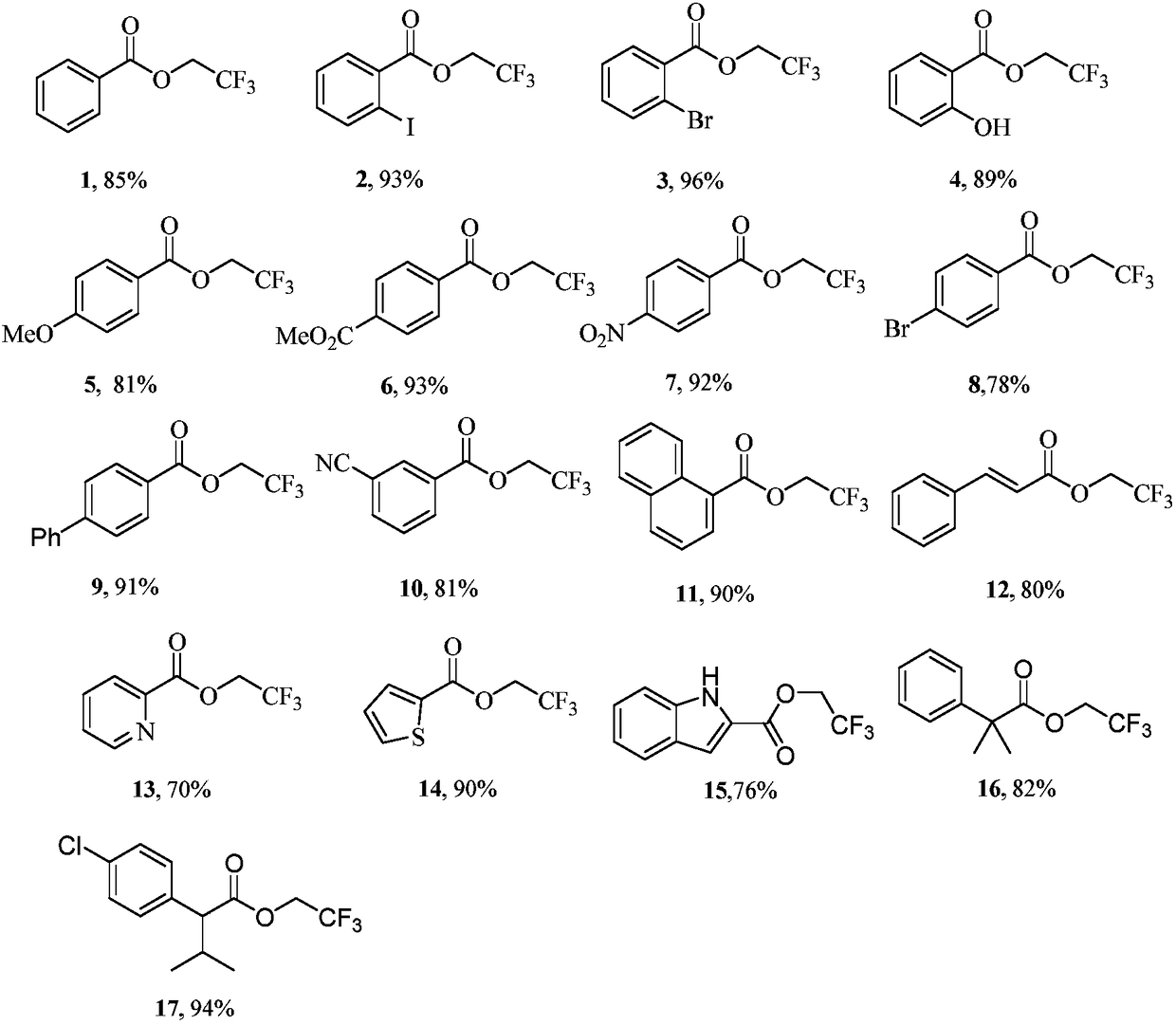
Embodiment 1~17
[0035] The following implementations 1-17 are prepared according to the following synthetic route, and compounds 1-17 can be prepared:
[0036]
[0037] The specific operation is: dissolve the aromatic carboxylic acid substrate (0.5mmol, 1equiv) in 1,1,1,3,3,3-hexafluoro-2-propanol (3ml), add dropwise tert-butyl nitrite ( 150μL, 1.25mmol, 2.5equiv) and 2,2,2-trifluoroethylamine (100μL, 1.25mmol, 2.5equiv), stirred at room temperature for 1-10h, the solution in the reaction system was spin-dried under reduced pressure, and the residue Purify by column chromatography to obtain aromatic carboxylic acid trifluoroethyl ester compounds.
[0038] The pharmaceutical reagents used in the examples of the present invention were purchased from Anaiji Chemical Company.
[0039] Structural characterization of the compound:
[0040] Compound structures were determined by nuclear magnetic resonance (NMR) or mass spectroscopy (MS). The mensuration of NMR is to use BrukerAVANCE-400 or Var...
Embodiment 1
[0046] Benzoic acid (61 mg, 0.5 mmol, 1 equiv) was dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (3 ml); tert-butyl nitrite (150 μL, 1.25 mmol, 2.5 equiv ) and trifluoroethylamine (100 μL, 1.25 mmol, 2.5 equiv) were stirred evenly, and reacted at room temperature for 10 hours. After the reaction was complete, spin-dried under reduced pressure, and separated by silica gel flash column chromatography to obtain the target compound 1.
[0047] Substrate: Benzoic acid
[0048] product:
[0049] Compound 1: colorless oil (87mg, 85% yield); 1 H NMR (400MHz, CDCl 3 )δ8.08–7.94(m,2H),7.62–7.50(m,1H),7.40(t,J=7.8Hz,2H),4.62(q,J=8.4Hz,2H); 13 C NMR (100MHz, CDCl 3 )δ163.9, 132.9, 129.0, 127.6, 127.3, 122.1 (q, J = 277.2Hz), 59.8 (q, J = 36.7Hz); 19 FNMR (376MHz, CDCl 3 )δ-73.68(t, J=8.6Hz); 19 F { 1 H}NMR (376MHz, CDCl 3 )δ-73.68.
Embodiment 2
[0051] Dissolve o-iodobenzoic acid (124 mg, 0.5 mmol, 1 equiv) in 1,1,1,3,3,3-hexafluoro-2-propanol (3 ml); add tert-butyl nitrite (150 μL, 1.25 mmol, 2.5 equiv) and trifluoroethylamine (100 μL, 1.25 mmol, 2.5 equiv) were stirred evenly, and reacted at room temperature for 10 hours. After the reaction was complete, spin-dried under reduced pressure, and separated by silica gel flash column chromatography to obtain the target compound 2.
[0052] Substrate: o-iodobenzoic acid
[0053] product:
[0054] Compound 2: colorless oil (154mg, 83% yield); 1 H NMR (400MHz, CDCl 3 )δ7.96(dd, J=8.0, 1.2Hz, 1H), 7.82(dd, J=7.8, 1.7Hz, 1H), 7.36(td, J=7.6, 1.2Hz, 1H), 7.13(td, J =7.7,1.7Hz, 1H), 4.63(q, J=8.3Hz, 2H); 13 C NMR (100MHz, CDCl 3 )δ163.4, 140.8, 132.5, 131.8, 130.6, 127.1, 122.0 (q, J = 277.3Hz), 93.6, 60.1 (q, J = 36.9Hz); 19 F NMR (376MHz, CDCl 3 )δ-73.31 (t, J=8.3Hz); 19 F{ 1 H}NMR (376MHz, CDCl 3 )δ-73.31; HRMS (ESI) m / z calcdfor C 9 h 7 f 3 IO 2 + ,[M+H] +...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com